Toward a molecular pathogenic pathway for Yersinia pestis YopM
- PMID: 23248776
- PMCID: PMC3518861
- DOI: 10.3389/fcimb.2012.00155
Toward a molecular pathogenic pathway for Yersinia pestis YopM
Abstract
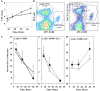
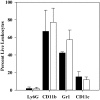
YopM is one of the six "effector Yops" of the human-pathogenic Yersinia, but its mechanism has not been defined. After delivery to J774A.1 monocyte-like cells, YopM can rapidly bind and activate the serine/threonine kinases RSK1 and PRK2. However, in infected mice, effects of Y. pestis YopM have been seen only after 24-48 h post-infection (p.i.). To identify potential direct effects of YopM in-vivo we tested for effects of YopM at 1 h and 16-18 h p.i. in mice infected systemically with 10(6) bacteria. At 16 h p.i., there was a robust host response to both parent and ΔyopM-1 Y. pestis KIM5. Compared to cells from non-infected mice, CD11b(+) cells from spleens of infected mice produced more than 100-fold greater IFNγ. In the corresponding sera there were more than 100-fold greater amounts of IFNγ, G-CSF, and CXCL9, as well as more than 10-fold greater amounts of IL-6, CXCL10, and CXCL1. The only YopM-related differences were slightly lower CXCL10 and IL-6 in sera from mice infected 16 h with parent compared to ΔyopM-1 Y. pestis. Microarray analysis of the CD11b(+) cells did not identify consistent transcriptional differences of ≥4-fold at 18 h p.i. However, at 1 h p.i. mRNA for early growth response transcription factor 1 (Egr1) was decreased when YopM was present. Bone marrow-derived macrophages infected for 1 h also expressed lower Egr1 message when YopM was present. Infected J774A.1 cells showed greater expression of Egr1 at 1 h p.i. when YopM was present, but this pattern reversed at 3 h. At 6 h p.i., Cxcl10 mRNA was lower in parent-strain infected cells. We conclude that decreased Egr1 expression is a very early transcriptional effect of YopM and speculate that a pathway may exist from RSK1 through Egr1. These studies revealed novel early transcriptional effects of YopM but point to a time after 18 h of infection when critical transitional events lead to later major effects on cytokine gene transcription.
Keywords: Yersinia; YopM; chemokine; microarray; plague.
Figures

References
-
- Becker R. A., Chambers J. M., Wilks A. R. (1988). The New S Language. New York, NY: Chapman and Hall
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

