Vitamin C promotes maturation of T-cells
- PMID: 23249337
- PMCID: PMC3869442
- DOI: 10.1089/ars.2012.4988
Vitamin C promotes maturation of T-cells
Abstract
Aims: Vitamin C (ascorbic acid) is thought to enhance immune function, but the mechanisms involved are obscure. We utilized an in vitro model of T-cell maturation to evaluate the role of ascorbic acid in lymphocyte development.
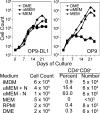
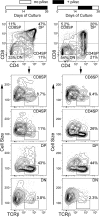
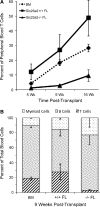
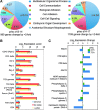
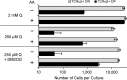
Results: Ascorbic acid was essential for the developmental progression of mouse bone marrow-derived progenitor cells to functional T-lymphocytes in vitro and also played a role in vivo. Ascorbate-mediated enhancement of T-cell development was lymphoid cell-intrinsic and independent of T-cell receptor (TCR) rearrangement. Analysis of TCR rearrangements demonstrated that ascorbic acid enhanced the selection of functional TCRαβ after the stage of β-selection. Genes encoding the coreceptor CD8 as well as the kinase ZAP70 were upregulated by ascorbic acid. Pharmacologic inhibition of methylation marks on DNA and histones enhanced ascorbate-mediated differentiation, suggesting an epigenetic mechanism of Cd8 gene regulation via active demethylation by ascorbate-dependent Fe(2+) and 2-oxoglutarate-dependent dioxygenases.
Innovation: We speculate that one aspect of gene regulation mediated by ascorbate occurs at the level of chromatin demethylation, mediated by Jumonji C (JmjC) domain enzymes that are known to be reliant upon ascorbate as a cofactor. JmjC domain enzymes are also known to regulate transcription factor activity. These two mechanisms are likely to play key roles in the modulation of immune development and function by ascorbic acid.
Conclusion: Our results provide strong experimental evidence supporting a role for ascorbic acid in T-cell maturation as well as insight into the mechanism of ascorbate-mediated enhancement of immune function.
Figures





References
-
- Arkan MC, Leonarduzzi G, Biasi F, Basaga H, and Poli G. Physiological amounts of ascorbate potentiate phorbol ester-induced nuclear-binding of AP-1 transcription factor in cells of macrophagic lineage. Free Radic Biol Med 31: 374–382, 2001 - PubMed
-
- Bergeron S, Anderson DK, and Swanson PC. RAG and HMGB1 proteins: purification and biochemical analysis of recombination signal complexes. Methods Enzymol 408: 511–528, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

