Over-expression of mitochondrial antiviral signaling protein inhibits coxsackievirus B3 infection by enhancing type-I interferons production
- PMID: 23249700
- PMCID: PMC3546859
- DOI: 10.1186/1743-422X-9-312
Over-expression of mitochondrial antiviral signaling protein inhibits coxsackievirus B3 infection by enhancing type-I interferons production
Abstract
Background: Recent studies have revealed that Mitochondrial Antiviral Signaling (MAVS) protein plays an essential role in the inhibition of viral infection through type I interferon (IFN) pathway. It has been shown that 3C (pro) cysteine protease of coxsackievirus B3 (CVB3) cleaves MAVS to inhibit type I IFNs induction. Other workers also found that MAVS knock-out mice suffered CVB3 susceptibility and severe histopathological change. Accordingly,our experiments were designed to explore the protection of over-expressing MAVS against CVB3 infection and the possible mechanism.
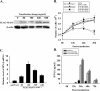
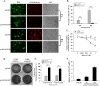
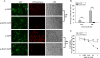
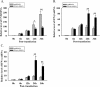
Results: In this study, HeLa cells (transfected with MAVS constructs pre- or post- exposure to CVB3) were used to analyze the function of exogenous MAVS on CVB3 infection. The results revealed that though CVB3 infection induced production of type I IFNs, viral replication and cell death were not effectively inhibited. Similarly, exogenous MAVS increased type I IFNs moderately. Morever, we observed robust production of type I IFNs in CVB3 post-infected HeLa cells thereby successfully inhibiting CVB3 infection, as well formation of cytopathic effect (CPE) and cell death. Finally, introduction of exogenous MAVS into CVB3 pre-infected cells also restricted viral infection efficiently by greatly up-regulating IFNs.
Conclusions: In summary, exogenous MAVS effectively prevents and controls CVB3 infection by modulating and promoting the production of type I IFNs. The IFNs level in MAVS over-expressing cells is still tightly regulated by CVB3 infection. Thus, the factors that up-regulate MAVS might be an alternative prescription in CVB3-related syndromes by enhancing IFNs production.
Figures

Similar articles
-
TRIM21 Restricts Coxsackievirus B3 Replication, Cardiac and Pancreatic Injury via Interacting With MAVS and Positively Regulating IRF3-Mediated Type-I Interferon Production.Front Immunol. 2018 Oct 25;9:2479. doi: 10.3389/fimmu.2018.02479. eCollection 2018. Front Immunol. 2018. PMID: 30410495 Free PMC article.
-
The coxsackievirus B 3C protease cleaves MAVS and TRIF to attenuate host type I interferon and apoptotic signaling.PLoS Pathog. 2011 Mar;7(3):e1001311. doi: 10.1371/journal.ppat.1001311. Epub 2011 Mar 10. PLoS Pathog. 2011. PMID: 21436888 Free PMC article.
-
Enterovirus 2Apro targets MDA5 and MAVS in infected cells.J Virol. 2014 Mar;88(6):3369-78. doi: 10.1128/JVI.02712-13. Epub 2014 Jan 3. J Virol. 2014. PMID: 24390337 Free PMC article.
-
c-FLIP-Short reduces type I interferon production and increases viremia with coxsackievirus B3.PLoS One. 2014 May 9;9(5):e96156. doi: 10.1371/journal.pone.0096156. eCollection 2014. PLoS One. 2014. PMID: 24816846 Free PMC article.
-
A Kidnapping Story: How Coxsackievirus B3 and Its Host Cell Interact.Cell Physiol Biochem. 2019;53(1):121-140. doi: 10.33594/000000125. Cell Physiol Biochem. 2019. PMID: 31230428 Review.
Cited by
-
Profiling Subcellular Protein Phosphatase Responses to Coxsackievirus B3 Infection of Cardiomyocytes.Mol Cell Proteomics. 2017 Apr;16(4 suppl 1):S244-S262. doi: 10.1074/mcp.O116.063487. Epub 2017 Feb 7. Mol Cell Proteomics. 2017. PMID: 28174228 Free PMC article.
-
Enteroviruses: A Gut-Wrenching Game of Entry, Detection, and Evasion.Viruses. 2019 May 21;11(5):460. doi: 10.3390/v11050460. Viruses. 2019. PMID: 31117206 Free PMC article. Review.
-
Revitalizing myocarditis treatment through gut microbiota modulation: unveiling a promising therapeutic avenue.Front Cell Infect Microbiol. 2023 May 16;13:1191936. doi: 10.3389/fcimb.2023.1191936. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37260696 Free PMC article. Review.
-
Inhibition of Type III Interferon Expression in Intestinal Epithelial Cells-A Strategy Used by Coxsackie B Virus to Evade the Host's Innate Immune Response at the Primary Site of Infection?Microorganisms. 2021 Jan 5;9(1):105. doi: 10.3390/microorganisms9010105. Microorganisms. 2021. PMID: 33466313 Free PMC article.
-
The antiviral effect of jiadifenoic acids C against coxsackievirus B3.Acta Pharm Sin B. 2014 Aug;4(4):277-83. doi: 10.1016/j.apsb.2014.06.008. Epub 2014 Jul 16. Acta Pharm Sin B. 2014. PMID: 26579396 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

