Differential lumbar spinal cord responses among wild type, CD4 knockout, and CD40 knockout mice in spinal nerve L5 transection-induced neuropathic pain
- PMID: 23249743
- PMCID: PMC3545955
- DOI: 10.1186/1744-8069-8-88
Differential lumbar spinal cord responses among wild type, CD4 knockout, and CD40 knockout mice in spinal nerve L5 transection-induced neuropathic pain
Abstract
Background: Our previous studies have indicated that both lumbar spinal cord-infiltrating CD4+ T cells and microglial CD40 contribute to the maintenance of mechanical hypersensitivity in a murine model of neuropathic pain spinal nerve L5 transection (L5Tx). To further delineate the CD4 and CD40-mediated mechanisms involved in the development of L5Tx-induced neuropathic pain behaviors, we examined the lumbar spinal cord mononuclear cells of wild type (WT) BALB/c, BALB/c-CD4 knockout (KO), and BALB/c-CD40 KO mice via flow cytometry.
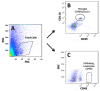
Results: In WT mice, L5Tx induced significant but transient (at day 3 and/or day 7) increases of the total numbers of mononuclear cells, microglial cells (CD45loCD11b+), and infiltrating leukocytes (CD45hi) in the ipsilateral side of the spinal cord. In CD4 KO mice, significant elevation of microglia was detected only on day 7 post-L5Tx, while no significant increase in infiltrating leukocytes post-L5Tx was observed. CD40 KO mice did not exhibit any of the changes observed in WT mice. Furthermore, neutralizing CD40 antibody treatment indicated an early involvement of CD40 signaling in the development of L5Tx-induced mechanical hypersensitivity.
Conclusions: Altogether, data indicate that both CD4 and CD40 play a role in L5Tx-induced leukocyte infiltration into the lumbar spinal cord but have differential contributions to spinal cord microglial activation following peripheral nerve injury.
Figures
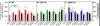
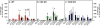
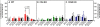

References
-
- Lindenlaub T, Sommer C. Cytokines in sural nerve biopsies from inflammatory and non-inflammatory neuropathies. Acta Neuropathol. 2003;105(6):593–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

