Detrimental effects of diet-induced obesity on τ pathology are independent of insulin resistance in τ transgenic mice
- PMID: 23250356
- PMCID: PMC3636620
- DOI: 10.2337/db12-0866
Detrimental effects of diet-induced obesity on τ pathology are independent of insulin resistance in τ transgenic mice
Abstract
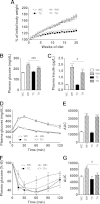
The τ pathology found in Alzheimer disease (AD) is crucial in cognitive decline. Midlife development of obesity, a major risk factor of insulin resistance and type 2 diabetes, increases the risk of dementia and AD later in life. The impact of obesity on AD risk has been suggested to be related to central insulin resistance, secondary to peripheral insulin resistance. The effects of diet-induced obesity (DIO) on τ pathology remain unknown. In this study, we evaluated effects of a high-fat diet, given at an early pathological stage, in the THY-Tau22 transgenic mouse model of progressive AD-like τ pathology. We found that early and progressive obesity potentiated spatial learning deficits as well as hippocampal τ pathology at a later stage. Surprisingly, THY-Tau22 mice did not exhibit peripheral insulin resistance. Further, pathological worsening occurred while hippocampal insulin signaling was upregulated. Together, our data demonstrate that DIO worsens τ phosphorylation and learning abilities in τ transgenic mice independently from peripheral/central insulin resistance.
Figures



Comment in
-
Does obesity-induced τ phosphorylation tip the scale toward dementia?Diabetes. 2013 May;62(5):1365-6. doi: 10.2337/db12-1784. Diabetes. 2013. PMID: 23613550 Free PMC article. No abstract available.
References
-
- Sergeant N, Bretteville A, Hamdane M, et al. Biochemistry of Tau in Alzheimer’s disease and related neurological disorders. Expert Rev Proteomics 2008;5:207–224 - PubMed
-
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 2011;70:960–969 - PubMed
-
- Grober E, Dickson D, Sliwinski MJ, et al. Memory and mental status correlates of modified Braak staging. Neurobiol Aging 1999;20:573–579 - PubMed
-
- Frisardi V, Solfrizzi V, Seripa D, et al. Metabolic-cognitive syndrome: a cross-talk between metabolic syndrome and Alzheimer’s disease. Ageing Res Rev 2010;9:399–417 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

