Androgens promote prostate cancer cell growth through induction of autophagy
- PMID: 23250485
- PMCID: PMC3683804
- DOI: 10.1210/me.2012-1260
Androgens promote prostate cancer cell growth through induction of autophagy
Abstract
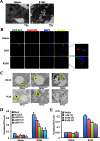
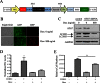
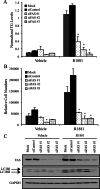
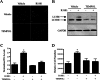
Androgens regulate both the physiological development of the prostate and the pathology of prostatic diseases. However, the mechanisms by which androgens exert their regulatory activities on these processes are poorly understood. In this study, we have determined that androgens regulate overall cell metabolism and cell growth, in part, by increasing autophagy in prostate cancer cells. Importantly, inhibition of autophagy using either pharmacological or molecular inhibitors significantly abrogated androgen-induced prostate cancer cell growth. Mechanistically, androgen-mediated autophagy appears to promote cell growth by augmenting intracellular lipid accumulation, an effect previously demonstrated to be necessary for prostate cancer cell growth. Further, autophagy and subsequent cell growth is potentiated, in part, by androgen-mediated increases in reactive oxygen species. These findings demonstrate a role for increased fat metabolism and autophagy in prostatic neoplasias and highlight the potential of targeting underexplored metabolic pathways for the development of novel therapeutics.
Figures



References
-
- Culig Z, Bartsch G. 2006. Androgen axis in prostate cancer. J Cell Biochem 99:373–381 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

