Translational pharmacokinetic-pharmacodynamic modeling from nonclinical to clinical development: a case study of anticancer drug, crizotinib
- PMID: 23250669
- PMCID: PMC3675733
- DOI: 10.1208/s12248-012-9436-4
Translational pharmacokinetic-pharmacodynamic modeling from nonclinical to clinical development: a case study of anticancer drug, crizotinib
Abstract
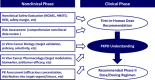
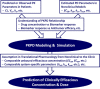
Attrition risk related to efficacy is still a major reason why new chemical entities fail in clinical trials despite recently increased understanding of translational pharmacology. Pharmacokinetic-pharmacodynamic (PKPD) analysis is key to translating in vivo drug potency from nonclinical models to patients by providing a quantitative assessment of in vivo drug potency with mechanistic insight of drug action. The pharmaceutical industry is clearly moving toward more mechanistic and quantitative PKPD modeling to have a deeper understanding of translational pharmacology. This paper summarizes an anticancer drug case study describing the translational PKPD modeling of crizotinib, an orally available, potent small molecule inhibitor of multiple tyrosine kinases including anaplastic lymphoma kinase (ALK) and mesenchymal-epithelial transition factor (MET), from nonclinical to clinical development. Overall, the PKPD relationships among crizotinib systemic exposure, ALK or MET inhibition, and tumor growth inhibition (TGI) in human tumor xenograft models were well characterized in a quantitative manner using mathematical modeling: the results suggest that 50% ALK inhibition is required for >50% TGI whereas >90% MET inhibition is required for >50% TGI. Furthermore, >75% ALK inhibition and >95% MET inhibition in patient tumors were projected by PKPD modeling during the clinically recommended dosing regimen, twice daily doses of crizotinib 250 mg (500 mg/day). These simulation results of crizotinib-mediated ALK and MET inhibition appeared consistent with the currently reported clinical responses. In summary, the present paper presents an anticancer drug example to demonstrate that quantitative PKPD modeling can be used for predictive translational pharmacology from nonclinical to clinical development.
Figures



Similar articles
-
Mechanistic understanding of translational pharmacokinetic-pharmacodynamic relationships in nonclinical tumor models: a case study of orally available novel inhibitors of anaplastic lymphoma kinase.Drug Metab Dispos. 2015 Jan;43(1):54-62. doi: 10.1124/dmd.114.061143. Epub 2014 Oct 27. Drug Metab Dispos. 2015. PMID: 25349124
-
Pharmacokinetic/pharmacodynamic modeling of crizotinib for anaplastic lymphoma kinase inhibition and antitumor efficacy in human tumor xenograft mouse models.J Pharmacol Exp Ther. 2012 Mar;340(3):549-57. doi: 10.1124/jpet.111.188870. Epub 2011 Nov 30. J Pharmacol Exp Ther. 2012. PMID: 22129595
-
Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children's Oncology Group phase 1 consortium study.Lancet Oncol. 2013 May;14(6):472-80. doi: 10.1016/S1470-2045(13)70095-0. Epub 2013 Apr 16. Lancet Oncol. 2013. PMID: 23598171 Free PMC article. Clinical Trial.
-
Crizotinib, a small-molecule dual inhibitor of the c-Met and ALK receptor tyrosine kinases.Curr Opin Investig Drugs. 2010 Dec;11(12):1477-90. Curr Opin Investig Drugs. 2010. PMID: 21154129 Review.
-
Crizotinib for the treatment of patients with advanced non-small cell lung cancer.Drugs Today (Barc). 2012 Apr;48(4):271-82. doi: 10.1358/dot.2012.48.4.1769835. Drugs Today (Barc). 2012. PMID: 22536569 Review.
Cited by
-
A Microfluidic Perfusion Platform for In Vitro Analysis of Drug Pharmacokinetic-Pharmacodynamic (PK-PD) Relationships.AAPS J. 2020 Mar 2;22(2):53. doi: 10.1208/s12248-020-0430-y. AAPS J. 2020. PMID: 32124093
-
Prediction of trough concentration and ALK occupancy in plasma and cerebrospinal fluid using physiologically based pharmacokinetic modeling of crizotinib, alectinib, and lorlatinib.Front Pharmacol. 2023 Nov 22;14:1234262. doi: 10.3389/fphar.2023.1234262. eCollection 2023. Front Pharmacol. 2023. PMID: 38074113 Free PMC article.
-
Translation of anticancer efficacy from nonclinical models to the clinic.CPT Pharmacometrics Syst Pharmacol. 2014 Aug 6;3(8):e128. doi: 10.1038/psp.2014.28. CPT Pharmacometrics Syst Pharmacol. 2014. PMID: 25098530 Free PMC article.
-
Practical Recommendations for Therapeutic Drug Monitoring of Kinase Inhibitors in Oncology.Clin Pharmacol Ther. 2017 Nov;102(5):765-776. doi: 10.1002/cpt.787. Epub 2017 Sep 7. Clin Pharmacol Ther. 2017. PMID: 28699160 Free PMC article. Review.
-
Catalytic Degraders Effectively Address Kinase Site Mutations in EML4-ALK Oncogenic Fusions.J Med Chem. 2023 Apr 27;66(8):5524-5535. doi: 10.1021/acs.jmedchem.2c01864. Epub 2023 Apr 10. J Med Chem. 2023. PMID: 37036171 Free PMC article.
References
-
- Derendorf H, Lesko LJ, Chaikin P, Colburn WA, Lee P, Miller R, et al. Pharmacokinetic/pharmacodynamic modeling in drug research and development. J Clin Pharmacol. 2000;40:1399–1418. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

