Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages
- PMID: 23250746
- PMCID: PMC3561515
- DOI: 10.1074/jbc.M112.419978
Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages
Abstract
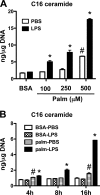
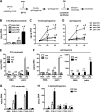
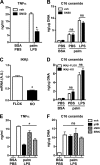
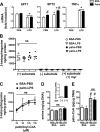
Macrophages play a key role in host defense and in tissue repair after injury. Emerging evidence suggests that macrophage dysfunction in states of lipid excess can contribute to the development of insulin resistance and may underlie inflammatory complications of diabetes. Ceramides are sphingolipids that modulate a variety of cellular responses including cell death, autophagy, insulin signaling, and inflammation. In this study we investigated the intersection between TLR4-mediated inflammatory signaling and saturated fatty acids with regard to ceramide generation. Primary macrophages treated with lipopolysaccharide (LPS) did not produce C16 ceramide, whereas palmitate exposure led to a modest increase in this sphingolipid. Strikingly, the combination of LPS and palmitate led to a synergistic increase in C16 ceramide. This response occurred via cross-talk at the level of de novo ceramide synthesis in the ER. The synergistic response required TLR4 signaling via MyD88 and TIR-domain-containing adaptor-inducing interferon beta (TRIF), whereas palmitate-induced ceramide production occurred independent of these inflammatory molecules. This ceramide response augmented IL-1β and TNFα release, a process that may contribute to the enhanced inflammatory response in metabolic diseases characterized by dyslipidemia.
Figures


References
-
- Schwartz E. A., Zhang W. Y., Karnik S. K., Borwege S., Anand V. R., Laine P. S., Su Y., Reaven P. D. (2010) Nutrient modification of the innate immune response. A novel mechanism by which saturated fatty acids greatly amplify monocyte inflammation. Arterioscler. Thromb. Vasc. Biol. 30, 802–808 - PubMed
-
- Cascio G., Schiera G., Di Liegro I. (2012) Dietary fatty acids in metabolic syndrome, diabetes, and cardiovascular diseases. Curr. Diabetes Rev. 8, 2–17 - PubMed
-
- Assimacopoulos-Jeannet F. (2004) Fat storage in pancreas and in insulin-sensitive tissues in pathogenesis of type 2 diabetes. Int. J. Obes. Relat. Metab. Disord. 28, S53–S57 - PubMed
-
- Joshi N., Caputo G. M., Weitekamp M. R., Karchmer A. W. (1999) Infections in patients with diabetes mellitus. N. Engl. J. Med. 341, 1906–1912 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

