The non-lysosomal β-glucosidase GBA2 is a non-integral membrane-associated protein at the endoplasmic reticulum (ER) and Golgi
- PMID: 23250757
- PMCID: PMC3561557
- DOI: 10.1074/jbc.M112.414714
The non-lysosomal β-glucosidase GBA2 is a non-integral membrane-associated protein at the endoplasmic reticulum (ER) and Golgi
Abstract
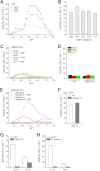
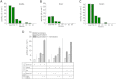
GBA1 and GBA2 are both β-glucosidases, which cleave glucosylceramide (GlcCer) to glucose and ceramide. GlcCer is a main precursor for higher order glycosphingolipids but might also serve as intracellular messenger. Mutations in the lysosomal GBA1 underlie Gaucher disease, the most common lysosomal storage disease in humans. Knocking out the non-lysosomal GBA2 in mice results in accumulation of GlcCer outside the lysosomes in various tissues (e.g. testis and liver) and impairs sperm development and liver regeneration. However, the underlying mechanisms are not well understood. To reveal the physiological function of GBA2 and, thereby, of the non-lysosomal GlcCer pool, it is important to characterize the localization of GBA2 and its activity in different tissues. Thus, we generated GBA2-specific antibodies and developed an assay that discriminates between GBA1 and GBA2 without the use of detergent. We show that GBA2 is not, as previously thought, an integral membrane protein but rather a cytosolic protein that tightly associates with cellular membranes. The interaction with the membrane, in particular with phospholipids, is important for its activity. GBA2 is localized at the ER and Golgi, which puts GBA2 in a key position for a lysosome-independent route of GlcCer-dependent signaling. Furthermore, our results suggest that GBA2 might affect the phenotype of Gaucher disease, because GBA2 activity is reduced in Gba1 knock-out fibroblasts and fibroblasts from a Gaucher patient. Our results provide the basis to understand the mechanism for GBA2 function in vivo and might help to unravel the role of GBA2 during pathogenesis of Gaucher disease.
Figures




References
-
- Paul P., Kamisaka Y., Marks D. L., Pagano R. E. (1996) Purification and characterization of UDP-glucose:ceramide glucosyltransferase from rat liver Golgi membranes. J. Biol. Chem. 271, 2287–2293 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

