The B subunit of an AB5 toxin produced by Salmonella enterica serovar Typhi up-regulates chemokines, cytokines, and adhesion molecules in human macrophage, colonic epithelial, and brain microvascular endothelial cell lines
- PMID: 23250951
- PMCID: PMC3584882
- DOI: 10.1128/IAI.01043-12
The B subunit of an AB5 toxin produced by Salmonella enterica serovar Typhi up-regulates chemokines, cytokines, and adhesion molecules in human macrophage, colonic epithelial, and brain microvascular endothelial cell lines
Abstract
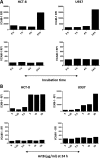
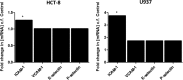
The principal function of bacterial AB5 toxin B subunits is to interact with glycan receptors on the surfaces of target cells and mediate the internalization of holotoxin. However, B subunit-receptor interactions also have the potential to impact cell signaling pathways and, in so doing, contribute to pathogenesis independently of the catalytic (toxic) A subunits. Various Salmonella enterica serovars, including Salmonella enterica serovar Typhi, encode an AB5 toxin (ArtAB), the A subunit of which is an ADP-ribosyltransferase related to the S1 subunit of pertussis toxin. However, although the A subunit is able to catalyze ADP-ribosylation of host G proteins, a cytotoxic phenotype has yet to be identified for the holotoxin. We therefore examined the capacity of the purified B subunit (ArtB) from S. Typhi to elicit cytokine, chemokine, and adhesion molecule responses in human macrophage (U937), colonic epithelial (HCT-8) cell, and brain microvascular endothelial cell (HBMEC) lines. Secretion of the chemokines monocyte chemotactic protein 1 (MCP-1) and interleukin 8 (IL-8) was increased in all three tested cell lines, with macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and granulocyte colony-stimulating factor (G-CSF) also significantly increased in U937 cells. ArtB also upregulated the cytokines tumor necrosis factor alpha (TNF-α) and IL-6 in HBMECs and HCT-8 cells, but not in U937 cells, while intercellular adhesion molecule 1 (ICAM-1) was upregulated in HCT-8 and U937 cells and vascular cell adhesion molecule 1 (VCAM-1) was upregulated in HBMECs. Thus, ArtB may contribute to pathogenesis independently of the A subunit by promoting and maintaining a strong inflammatory response at the site of infection.
Figures






References
-
- Paton AW, Beddoe T, Thorpe CM, Whisstock JC, Wilce MC, Rossjohn J, Talbot UM, Paton JC. 2006. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature 443:548–552 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

