AKT2 confers protection against aortic aneurysms and dissections
- PMID: 23250987
- PMCID: PMC3586338
- DOI: 10.1161/CIRCRESAHA.112.300735
AKT2 confers protection against aortic aneurysms and dissections
Abstract
Rationale: Aortic aneurysm and dissection (AAD) are major diseases of the adult aorta caused by progressive medial degeneration of the aortic wall. Although the overproduction of destructive factors promotes tissue damage and disease progression, the role of protective pathways is unknown.
Objective: In this study, we examined the role of AKT2 in protecting the aorta from developing AAD.
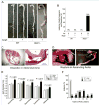
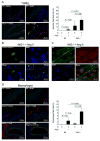
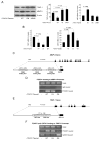
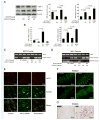
Methods and results: AKT2 and phospho-AKT levels were significantly downregulated in human thoracic AAD tissues, especially within the degenerative medial layer. Akt2-deficient mice showed abnormal elastic fibers and reduced medial thickness in the aortic wall. When challenged with angiotensin II, these mice developed aortic aneurysm, dissection, and rupture with features similar to those in humans, in both thoracic and abdominal segments. Aortas from Akt2-deficient mice displayed profound tissue destruction, apoptotic cell death, and inflammatory cell infiltration that were not observed in aortas from wild-type mice. In addition, angiotensin II-infused Akt2-deficient mice showed significantly elevated expression of matrix metalloproteinase-9 (MMP-9) and reduced expression of tissue inhibitor of metalloproteinase-1 (TIMP-1). In cultured human aortic vascular smooth muscle cells, AKT2 inhibited the expression of MMP-9 and stimulated the expression of TIMP-1 by preventing the binding of transcription factor forkhead box protein O1 to the MMP-9 and TIMP-1 promoters.
Conclusions: Impaired AKT2 signaling may contribute to increased susceptibility to the development of AAD. Our findings provide evidence of a mechanism that underlies the protective effects of AKT2 on the aortic wall and that may serve as a therapeutic target in the prevention of AAD.
Figures




Comment in
-
Molecular mechanisms behind aneurysm and dissection formation.Neurosurgery. 2013 Dec;73(6):N10-1. doi: 10.1227/01.neu.0000438330.64943.51. Neurosurgery. 2013. PMID: 24257335 No abstract available.
References
-
- Schlatmann TJ, Becker AE. Pathogenesis of dissecting aneurysm of aorta. Comparative histopathologic study of significance of medial changes. Am J Cardiol. 1977;39:21–26. - PubMed
-
- Shiojima I, Walsh K. Role of akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

