Gastrin-releasing peptide receptors in the central nervous system: role in brain function and as a drug target
- PMID: 23251133
- PMCID: PMC3523293
- DOI: 10.3389/fendo.2012.00159
Gastrin-releasing peptide receptors in the central nervous system: role in brain function and as a drug target
Abstract
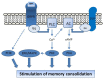
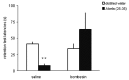
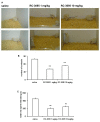
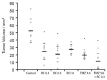
Neuropeptides acting on specific cell membrane receptors of the G protein-coupled receptor (GPCR) superfamily regulate a range of important aspects of nervous and neuroendocrine function. Gastrin-releasing peptide (GRP) is a mammalian neuropeptide that binds to the GRP receptor (GRPR, BB2). Increasing evidence indicates that GRPR-mediated signaling in the central nervous system (CNS) plays an important role in regulating brain function, including aspects related to emotional responses, social interaction, memory, and feeding behavior. In addition, some alterations in GRP or GRPR expression or function have been described in patients with neurodegenerative, neurodevelopmental, and psychiatric disorders, as well as in brain tumors. Findings from preclinical models are consistent with the view that the GRPR might play a role in brain disorders, and raise the possibility that GRPR agonists might ameliorate cognitive and social deficits associated with neurological diseases, while antagonists may reduce anxiety and inhibit the growth of some types of brain cancer. Further preclinical and translational studies evaluating the potential therapeutic effects of GRPR ligands are warranted.
Keywords: bombesin receptors; brain disorders; gastrin-releasing peptide; gastrin-releasing peptide receptor; neuropeptide signaling.
Figures

Similar articles
-
Gastrin-releasing peptide receptor signaling in the integration of stress and memory.Neurobiol Learn Mem. 2014 Jul;112:44-52. doi: 10.1016/j.nlm.2013.08.013. Epub 2013 Aug 31. Neurobiol Learn Mem. 2014. PMID: 24001571 Review.
-
The gastrin-releasing peptide receptor as a therapeutic target in central nervous system disorders.Recent Pat CNS Drug Discov. 2007 Jun;2(2):125-9. doi: 10.2174/157488907780832742. Recent Pat CNS Drug Discov. 2007. PMID: 18221223
-
Gastrin-releasing peptide receptor as a molecular target for psychiatric and neurological disorders.CNS Neurol Disord Drug Targets. 2006 Apr;5(2):197-204. doi: 10.2174/187152706776359673. CNS Neurol Disord Drug Targets. 2006. PMID: 16611092 Review.
-
Targeting the gastrin-releasing peptide receptor pathway to treat cognitive dysfunctionassociated with Alzheimer's Disease.Dement Neuropsychol. 2007 Apr-Jun;1(2):118-123. doi: 10.1590/s1980-57642008dn10200002. Dement Neuropsychol. 2007. PMID: 29213377 Free PMC article. Review.
-
Novel insight on GRP/GRPR axis in diseases.Biomed Pharmacother. 2023 May;161:114497. doi: 10.1016/j.biopha.2023.114497. Epub 2023 Mar 16. Biomed Pharmacother. 2023. PMID: 36933382 Review.
Cited by
-
Combinatorial Expression of Grp and Neurod6 Defines Dopamine Neuron Populations with Distinct Projection Patterns and Disease Vulnerability.eNeuro. 2018 Jun 13;5(3):ENEURO.0152-18.2018. doi: 10.1523/ENEURO.0152-18.2018. eCollection 2018 May-Jun. eNeuro. 2018. PMID: 30135866 Free PMC article.
-
Transcriptomic profiling of high- and low-spiking regions reveals novel epileptogenic mechanisms in focal cortical dysplasia type II patients.Mol Brain. 2021 Jul 23;14(1):120. doi: 10.1186/s13041-021-00832-4. Mol Brain. 2021. PMID: 34301297 Free PMC article.
-
The Nonpeptide Agonist MK-5046 Functions As an Allosteric Agonist for the Bombesin Receptor Subtype-3.J Pharmacol Exp Ther. 2022 Aug;382(2):66-78. doi: 10.1124/jpet.121.001033. Epub 2022 May 29. J Pharmacol Exp Ther. 2022. PMID: 35644465 Free PMC article.
-
Hippocampal gene expression meta-analysis identifies aging and age-associated spatial learning impairment (ASLI) genes and pathways.PLoS One. 2013 Jul 18;8(7):e69768. doi: 10.1371/journal.pone.0069768. Print 2013. PLoS One. 2013. PMID: 23874995 Free PMC article.
-
Adaptation of Respiratory-Related Brain Regions to Long-Term Hypercapnia: Focus on Neuropeptides in the RTN.Front Neurosci. 2019 Dec 13;13:1343. doi: 10.3389/fnins.2019.01343. eCollection 2019. Front Neurosci. 2019. PMID: 31920508 Free PMC article.
References
-
- Abujamra A. L., Almeida V. R., Brunetto A. L., Schwartsmann G., Roesler R. (2009). A gastrin-releasing peptide receptor antagonist stimulates Neuro2a neuroblastoma cell growth: prevention by a histone deacetylase inhibitor. Cell Biol. Int. 33 899–903 - PubMed
-
- Battey J., Wada E. (1991). Two distinct receptor subtypes for mammalian bombesin-like peptides. Trends Neurosci. 14 524–528 - PubMed
-
- Bissette G., Nemeroff C. B., Decker M. W., Kizer J. S., Agid Y., Javoy-Agid F. (1985). Alterations in regional brain concentrations of neurotensin and bombesin in Parkinson’s disease. Ann. Neurol. 17 324–328 - PubMed
-
- Brown M., Rivier J., Vale W. (1977a). Bombesin: potent effects on thermoregulation in the rat. Science 196 998–1000 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

