Complex role of microRNAs in HTLV-1 infections
- PMID: 23251140
- PMCID: PMC3523292
- DOI: 10.3389/fgene.2012.00295
Complex role of microRNAs in HTLV-1 infections
Abstract
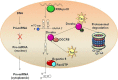
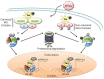
Human T-lymphotropic virus 1 (HTLV-1) was the first human retrovirus to be discovered and is the causative agent of adult T-cell leukemia/lymphoma (ATL) and the neurodegenerative disease HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). The importance of microRNA (miRNA) in the replicative cycle of several other viruses, as well as in the progression of associated pathologies, has been well established in the past decade. Moreover, involvement of miRNA alteration in the HTLV-1 life cycle, and in the progression of its related oncogenic and neurodegenerative diseases, has recently come to light. Several HTLV-1 derived proteins alter transcription factor functionalities, interact with chromatin remodelers, or manipulate components of the RNA interference (RNAi) machinery, thereby establishing various routes by which miRNA expression can be up- or down-regulated in the host cell. Furthermore, the mechanism of action through which dysregulation of host miRNAs affects HTLV-1 infected cells can vary substantially and include mRNA silencing via the RNA-induced silencing complex (RISC), transcriptional gene silencing, inhibition of RNAi components, and chromatin remodeling. These miRNA-induced changes can lead to increased cell survival, invasiveness, proliferation, and differentiation, as well as allow for viral latency. While many recent studies have successfully implicated miRNAs in the life cycle and pathogenesis of HTLV-1 infections, there are still significant outstanding questions to be addressed. Here we will review recent discoveries elucidating HTLV-1 mediated manipulation of host cell miRNA profiles and examine the impact on pathogenesis, as well as explore future lines of inquiry that could increase understanding in this field of study.
Keywords: ATL; HAM/TSP; HTLV-1; NF-κB; RNAi; Tax; chromatin; miRNA.
Figures

References
-
- Ahlenstiel C. L., Lim H. G., Cooper D. A., Ishida T., Kelleher A. D., Suzuki K. (2012). Direct evidence of nuclear Argonaute distribution during transcriptional silencing links the actin cytoskeleton to nuclear RNAi machinery in human cells. Nucleic Acids Res. 40, 1579–1595 10.1093/nar/gkr891 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

