Novel in vivo imaging analysis of an inner ear drug delivery system in mice: comparison of inner ear drug concentrations over time after transtympanic and systemic injections
- PMID: 23251331
- PMCID: PMC3520978
- DOI: 10.1371/journal.pone.0048480
Novel in vivo imaging analysis of an inner ear drug delivery system in mice: comparison of inner ear drug concentrations over time after transtympanic and systemic injections
Abstract
Objective: Systemic steroid injections are used to treat idiopathic sudden-onset sensorineural hearing loss (ISSHL) and some inner ear disorders. Recent studies show that transtympanic (TT) steroid injections are effective for treating ISSHL. As in vivo monitoring of drug delivery dynamics for inner ear is lacking, its time course and dispersion of drugs is unknown. Here, we used a new in vivo imaging system to monitor drug delivery in live mice and to compare drug concentrations over time after TT and systemic injections.
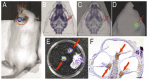
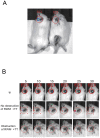
Methods: Luciferin delivered into the inner ears of GFAP-Luc transgenic mice reacted with luciferase in GFAP-expressing cells in the cochlear spiral ganglion, resulting in photon bioluminescence. We used the Xenogen IVIS® imaging system to measure how long photons continued to be emitted in the inner ear after TT or systemic injections of luciferin, and then compared the associated drug dynamics.
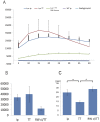
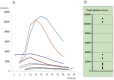
Results: The response to TT and IP injections differed significantly. Photons were detected five minutes after TT injection, peaking at ~20 minutes. By contrast, photons were first detected 30 minutes after i.p. injection. TT and i.p. drug delivery time differed considerably. With TT injections, photons were detected earlier than with IP injections. Photon bioluminescence also disappeared sooner. Delivery time varied with TT injections.
Conclusions: We speculate that the drug might enter the Eustachian tube from the middle ear. We conclude that inner-ear drug concentration can be maintained longer if the two injection routes are combined. As the size of luciferin differs from that of therapeutics like dexamethasone, combining drugs with luciferin may advance our understanding of in vivo drug delivery dynamics in the inner ear.
Conflict of interest statement
Figures
Similar articles
-
Novel in vivo imaging analysis of an inner ear drug delivery system: Drug availability in inner ear following different dose of systemic drug injections.Hear Res. 2015 Dec;330(Pt A):142-6. doi: 10.1016/j.heares.2015.09.018. Epub 2015 Oct 3. Hear Res. 2015. PMID: 26435094
-
Transtympanic perfusion: indications and limitations.Curr Opin Otolaryngol Head Neck Surg. 2004 Oct;12(5):378-83. doi: 10.1097/01.moo.0000134438.91734.38. Curr Opin Otolaryngol Head Neck Surg. 2004. PMID: 15377947 Review.
-
Treatment of sudden sensorineural hearing loss with transtympanic injection of steroids as single therapy: a randomized clinical study.Eur Arch Otorhinolaryngol. 2011 Sep;268(9):1273-8. doi: 10.1007/s00405-011-1523-0. Epub 2011 Feb 16. Eur Arch Otorhinolaryngol. 2011. PMID: 21327728 Clinical Trial.
-
Comparison of inner ear drug availability of combined treatment with systemic or local drug injections alone.Neurosci Res. 2020 Jun;155:27-33. doi: 10.1016/j.neures.2019.07.001. Epub 2019 Jul 3. Neurosci Res. 2020. PMID: 31278973
-
Intratympanic dexamethasone for sudden sensorineural hearing loss after failure of systemic therapy.Laryngoscope. 2007 Jan;117(1):3-15. doi: 10.1097/01.mlg.0000245058.11866.15. Laryngoscope. 2007. PMID: 17202923 Review.
Cited by
-
Transforming growth factor β1 inhibition protects from noise-induced hearing loss.Front Aging Neurosci. 2015 Mar 20;7:32. doi: 10.3389/fnagi.2015.00032. eCollection 2015. Front Aging Neurosci. 2015. PMID: 25852546 Free PMC article.
-
Sustained Effect of Hyaluronic Acid in Subcutaneous Administration to the Cochlear Spiral Ganglion.PLoS One. 2016 Apr 21;11(4):e0153957. doi: 10.1371/journal.pone.0153957. eCollection 2016. PLoS One. 2016. PMID: 27099926 Free PMC article.
-
In Vivo Bioluminescent Imaging of ATP-Binding Cassette Transporter-Mediated Efflux at the Blood-Brain Barrier.Methods Mol Biol. 2016;1461:227-39. doi: 10.1007/978-1-4939-3813-1_19. Methods Mol Biol. 2016. PMID: 27424909 Free PMC article.
-
Bioluminescent imaging of drug efflux at the blood-brain barrier mediated by the transporter ABCG2.Proc Natl Acad Sci U S A. 2013 Dec 17;110(51):20801-6. doi: 10.1073/pnas.1312159110. Epub 2013 Dec 2. Proc Natl Acad Sci U S A. 2013. PMID: 24297888 Free PMC article.
-
Impact of noise exposure on the circadian clock in the auditory system.J Acoust Soc Am. 2019 Nov;146(5):3960. doi: 10.1121/1.5132290. J Acoust Soc Am. 2019. PMID: 31795664 Free PMC article. Review.
References
-
- Rauch SD, Halpin CF, Antonelli PJ, Babu S, Carey JP, et al. (2011) Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA: the journal of the American Medical Association 305: 2071–2079. - PubMed
-
- Silverstein H, Rowan PT, Olds MJ, Rosenberg SI (1997) Inner ear perfusion and the role of round window patency. The American journal of otology 18: 586–589. - PubMed
-
- Kanzaki S, Saito H, Inoue Y, Ogawa K (2012) A new device for delivering drugs into the inner ear: Otoendoscope with microcatheter. Auris, nasus, larynx 39: 208–211. - PubMed
-
- Thorne M, Salt AN, DeMott JE, Henson MM, Henson OW Jr, et al. (1999) Cochlear fluid space dimensions for six species derived from reconstructions of three-dimensional magnetic resonance images. The Laryngoscope 109: 1661–1668. - PubMed
-
- Zhu L, Ramboz S, Hewitt D, Boring L, Grass DS, et al. (2004) Non-invasive imaging of GFAP expression after neuronal damage in mice. Neuroscience letters 367: 210–212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

