Obstruction of photoinduced electron transfer from excited porphyrin to graphene oxide: a fluorescence turn-on sensing platform for iron (III) ions
- PMID: 23251366
- PMCID: PMC3519470
- DOI: 10.1371/journal.pone.0050367
Obstruction of photoinduced electron transfer from excited porphyrin to graphene oxide: a fluorescence turn-on sensing platform for iron (III) ions
Abstract
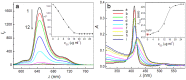
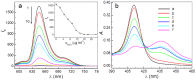
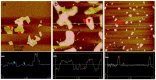
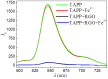
A comparative research of the assembly of different porphyrin molecules on graphene oxide (GO) and reduced graphene oxide (RGO) was carried out, respectively. Despite the cationic porphyrin molecules can be assembled onto the surfaces of graphene sheets, including GO and RGO, to form complexes through electrostatic and π-π stacking interactions, the more obvious fluorescence quenching and the larger red-shift of the Soret band of porphyrin molecule in RGO-bound states were observed than those in GO-bound states, due to the difference of molecular flattening in degree. Further, more interesting finding was that the complexes formed between cationic porphyrin and GO, rather than RGO sheets, can facilitate the incorporation of iron (III) ions into the porphyrin moieties, due to the presence of the oxygen-contained groups at the basal plane of GO sheets served as auxiliary coordination units, which can high-efficiently obstruct the electron transfer from excited porphyrin to GO sheets and result in the occurrence of fluorescence restoration. Thus, a fluorescence sensing platform has been developed for iron (III) ions detection in this contribution by using the porphyrin/GO nanohybrids as an optical probe, and our present one exhibited rapid and sensitive responses and high selectivity toward iron (III) ions.
Conflict of interest statement
Figures





References
-
- Li D, Kaner RB (2008) Graphene-based materials. Science 320: 1170–1171. - PubMed
-
- Dikin DA, Stankovich S, Zimney EJ, Piner RD, Dommett GHB, et al. (2007) Preparation and characterization of graphene oxide paper. Nature 448: 457–460. - PubMed
-
- Bunch SJ, van der Zande AM, Verbridge SS, Frank IW, Tanenbaum DM, et al. (2007) Electromechanical resonators from graphene sheets. Science 315: 490–493. - PubMed
-
- Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6: 183–191. - PubMed
-
- Xu YF, Liu ZB, Zhang XL, Wang Y, Tian JG, et al. (2009) A graphene hybrid material covalently functionalized with porphyrin: synthesis and optical limiting property. Adv Mater 21: 1275–1279.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

