Transgenic quail production by microinjection of lentiviral vector into the early embryo blood vessels
- PMID: 23251391
- PMCID: PMC3520935
- DOI: 10.1371/journal.pone.0050817
Transgenic quail production by microinjection of lentiviral vector into the early embryo blood vessels
Abstract
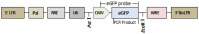
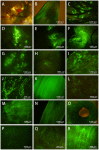
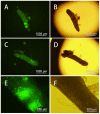
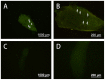
Several strategies have been used to generate transgenic birds. The most successful method so far has been the injection of lentiviral vectors into the subgerminal cavity of a newly laid egg. We report here a new, easy and effective way to produce transgenic quails through direct injection of a lentiviral vector, containing an enhanced-green fluorescent protein (eGFP) transgene, into the blood vessels of quail embryos at Hamburger-Hamilton stage 13-15 (HH13-15). A total of 80 embryos were injected and 48 G0 chimeras (60%) were hatched. Most injected embryo organs and tissues of hatched quails were positive for eGFP. In five out of 21 mature G0 male quails, the semen was eGFP-positive, as detected by polymerase chain reaction (PCR), indicating transgenic germ line chimeras. Testcross and genetic analyses revealed that the G0 quail produced transgenic G1 offspring; of 46 G1 hatchlings, 6 were transgenic (6/46, 13.0%). We also compared this new method with the conventional transgenesis using stage X subgerminal cavity injection. Total 240 quail embryos were injected by subgerminal cavity injection, of which 34 (14.1%) were hatched, significantly lower than the new method. From these hatched quails semen samples were collected from 19 sexually matured males and tested for the transgene by PCR. The transgene was present in three G0 male quails and only 4/236 G1 offspring (1.7%) were transgenic. In conclusion, we developed a novel bird transgenic method by injection of lentiviral vector into embryonic blood vessel at HH 13-15 stage, which result in significant higher transgenic efficiency than the conventional subgerminal cavity injection.
Conflict of interest statement
Figures


References
-
- Scott BB, Lois C (2006) Generation of transgenic birds with replication-deficient lentiviruses. Nature Protocol 1: 1406–1411. - PubMed
-
- Sang H (2004) Prospects for transgenesis in the chick. Mechanisms of Development 121: 1179–1186. - PubMed
-
- Carsience RS, Clark ME, Verrinder Gibbins AM, Etches RJ (1993) Germline chimeric chickens from dispersed donor blastodermal cells and compromised recipient embryos. Development 117: 669–675. - PubMed
-
- Han JY (2009) Germ cells and transgenesis in chickens. Comparative Immunology, Microbiology & Infectious Diseases 32: 61–80. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

