Exome sequencing identifies a founder frameshift mutation in an alternative exon of USH1C as the cause of autosomal recessive retinitis pigmentosa with late-onset hearing loss
- PMID: 23251578
- PMCID: PMC3520954
- DOI: 10.1371/journal.pone.0051566
Exome sequencing identifies a founder frameshift mutation in an alternative exon of USH1C as the cause of autosomal recessive retinitis pigmentosa with late-onset hearing loss
Abstract
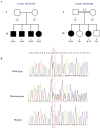
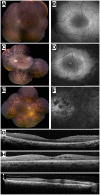
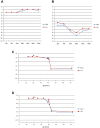
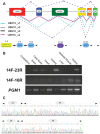
We used a combined approach of homozygosity mapping and whole exome sequencing (WES) to search for the genetic cause of autosomal recessive retinitis pigmentosa (arRP) in families of Yemenite Jewish origin. Homozygosity mapping of two arRP Yemenite Jewish families revealed a few homozygous regions. A subsequent WES analysis of the two index cases revealed a shared homozygous novel nucleotide deletion (c.1220delG) leading to a frameshift (p.Gly407Glufs*56) in an alternative exon (#15) of USH1C. Screening of additional Yemenite Jewish patients revealed a total of 16 homozygous RP patients (with a carrier frequency of 0.008 in controls). Funduscopic and electroretinography findings were within the spectrum of typical RP. While other USH1C mutations usually cause Usher type I (including RP, vestibular dysfunction and congenital deafness), audiometric screening of 10 patients who are homozygous for c.1220delG revealed that patients under 40 years of age had normal hearing while older patients showed mild to severe high tone sensorineural hearing loss. This is the first report of a mutation in a known USH1 gene that causes late onset rather than congenital sensorineural hearing loss. The c.1220delG mutation of USH1C accounts for 23% of RP among Yemenite Jewish patients in our cohort.
Conflict of interest statement
Figures
References
-
- Haim M (2002) Epidemiology of retinitis pigmentosa in Denmark. Acta Ophthalmol Scand Suppl: 1–34. - PubMed
-
- Bunker CH, Berson EL, Bromley WC, Hayes RP, Roderick TH (1984) Prevalence of retinitis pigmentosa in Maine. Am J Ophthalmol 97: 357–365. - PubMed
-
- Hartong DT, Berson EL, Dryja TP (2006) Retinitis pigmentosa. Lancet 368: 1795–1809. - PubMed
-
- Kaiserman N, Obolensky A, Banin E, Sharon D (2007) Novel USH2A mutations in Israeli patients with retinitis pigmentosa and Usher syndrome type 2. Arch Ophthalmol 125: 219–224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

