A potent anti-HB-EGF monoclonal antibody inhibits cancer cell proliferation and multiple angiogenic activities of HB-EGF
- PMID: 23251664
- PMCID: PMC3522611
- DOI: 10.1371/journal.pone.0051964
A potent anti-HB-EGF monoclonal antibody inhibits cancer cell proliferation and multiple angiogenic activities of HB-EGF
Abstract
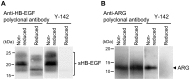
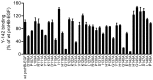
Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is a member of the epidermal growth factor family and has a variety of physiological and pathological functions. Modulation of HB-EGF activity might have a therapeutic potential in the oncology area. We explored the therapeutic possibilities by characterizing the in vitro biological activity of anti-HB-EGF monoclonal antibody Y-142. EGF receptor (EGFR) ligand and species specificities of Y-142 were tested. Neutralizing activities of Y-142 against HB-EGF were evaluated in EGFR and ERBB4 signaling. Biological activities of Y-142 were assessed in cancer cell proliferation and angiogenesis assays and compared with the anti-EGFR antibody cetuximab, the HB-EGF inhibitor CRM197, and the anti-vascular endothelial growth factor (VEGF) antibody bevacizumab. The binding epitope was determined with alanine scanning. Y-142 recognized HB-EGF as well as the EGFR ligand amphiregulin, and bound specifically to human HB-EGF, but not to rodent HB-EGF. In addition, Y-142 neutralized HB-EGF-induced phosphorylation of EGFR and ERBB4, and blocked their downstream ERK1/2 and AKT signaling. We also found that Y-142 inhibited HB-EGF-induced cancer cell proliferation, endothelial cell proliferation, tube formation, and VEGF production more effectively than cetuximab and CRM197 and that Y-142 was superior to bevacizumab in the inhibition of HB-EGF-induced tube formation. Six amino acids in the EGF-like domain were identified as the Y-142 binding epitope. Among the six amino acids, the combination of F115 and Y123 determined the amphiregulin cross-reactivity and that F115 accounted for the species selectivity. Furthermore, it was suggested that the potent neutralizing activity of Y-142 was derived from its recognition of R142 and Y123 and its high affinity to HB-EGF. Y-142 has a potent HB-EGF neutralizing activity that modulates multiple biological activities of HB-EGF including cancer cell proliferation and angiogenic activities. Y-142 may have a potential to be developed into a therapeutic agent for the treatment of HB-EGF-dependent cancers.
Conflict of interest statement
Figures



References
-
- Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M (1991) A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science 251: 936–939. - PubMed
-
- Higashiyama S, Lau K, Besner GE, Abraham JA, Klagsbrun M (1992) Structure of heparin-binding EGF-like growth factor. Multiple forms, primary structure, and glycosylation of the mature protein. J Biol Chem 267: 6205–6212. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

