Modelling dynamics in protein crystal structures by ensemble refinement
- PMID: 23251785
- PMCID: PMC3524795
- DOI: 10.7554/eLife.00311
Modelling dynamics in protein crystal structures by ensemble refinement
Abstract
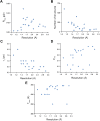
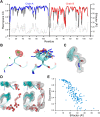
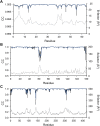
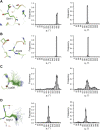
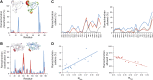
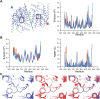
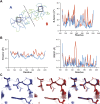
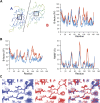
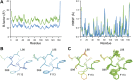
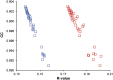
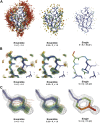
Single-structure models derived from X-ray data do not adequately account for the inherent, functionally important dynamics of protein molecules. We generated ensembles of structures by time-averaged refinement, where local molecular vibrations were sampled by molecular-dynamics (MD) simulation whilst global disorder was partitioned into an underlying overall translation-libration-screw (TLS) model. Modeling of 20 protein datasets at 1.1-3.1 Å resolution reduced cross-validated R(free) values by 0.3-4.9%, indicating that ensemble models fit the X-ray data better than single structures. The ensembles revealed that, while most proteins display a well-ordered core, some proteins exhibit a 'molten core' likely supporting functionally important dynamics in ligand binding, enzyme activity and protomer assembly. Order-disorder changes in HIV protease indicate a mechanism of entropy compensation for ordering the catalytic residues upon ligand binding by disordering specific core residues. Thus, ensemble refinement extracts dynamical details from the X-ray data that allow a more comprehensive understanding of structure-dynamics-function relationships.DOI:http://dx.doi.org/10.7554/eLife.00311.001.
Keywords: None; crystallography; dynamics; function; protein; structure.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR. 1984. Molecular dynamics with coupling to an external bath. The Journal of Chemical Physics 81:3684. 10.1063/1.448118 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

