G protein-coupled receptors--recent advances
- PMID: 23251911
- PMCID: PMC4322417
G protein-coupled receptors--recent advances
Abstract
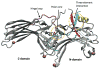
The years 2000 and 2007 witnessed milestones in current understanding of G protein-coupled receptor (GPCR) structural biology. In 2000 the first GPCR, bovine rhodopsin, was crystallized and the structure was solved, while in 2007 the structure of β(2)-adrenergic receptor, the first GPCR with diffusible ligands, was determined owing to advances in microcrystallization and an insertion of the fast-folding lysozyme into the receptor. In parallel with those crystallographic studies, the biological and biochemical characterization of GPCRs has advanced considerably because those receptors are molecular targets for many of currently used drugs. Therefore, the mechanisms of activation and signal transduction to the cell interior deduced from known GPCRs structures are of the highest importance for drug discovery. These proteins are the most diversified membrane receptors encoded by hundreds of genes in our genome. They participate in processes responsible for vision, smell, taste and neuronal transmission in response to photons or binding of ions, hormones, peptides, chemokines and other factors. Although the GPCRs share a common seven-transmembrane α-helical bundle structure their binding sites can accommodate thousands of different ligands. The ligands, including agonists, antagonists or inverse agonists change the structure of the receptor. With bound agonists they can form a complex with a suitable G protein, be phosphorylated by kinases or bind arrestin. The discovered signaling cascades invoked by arrestin independently of G proteins makes the GPCR activating scheme more complex such that a ligand acting as an antagonist for G protein signaling can also act as an agonist in arrestin-dependent signaling. Additionally, the existence of multiple ligand-dependent partial activation states as well as dimerization of GPCRs result in a 'microprocessor-like' action of these receptors rather than an 'on-off' switch as was commonly believed only a decade ago.
Figures
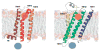






References
-
- Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. - PubMed
-
- Anor MED. Plerixafor: AMD 3100, AMD3100, JM 3100, SDZ SID 791. Drugs R D. 2007;8:113–119. - PubMed
-
- Arshavsky VY, Lamb TD, Pugh EN., Jr G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
