Light-induced subunit dissociation by a light-oxygen-voltage domain photoreceptor from Rhodobacter sphaeroides
- PMID: 23252338
- PMCID: PMC3582384
- DOI: 10.1021/bi3015373
Light-induced subunit dissociation by a light-oxygen-voltage domain photoreceptor from Rhodobacter sphaeroides
Abstract
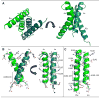
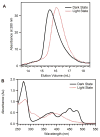
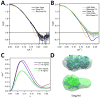
Light-oxygen-voltage (LOV) domains bind a flavin chromophore to serve as blue light sensors in a wide range of eukaryotic and prokaryotic proteins. LOV domains are associated with a variable effector domain or a separate protein signaling partner to execute a wide variety of functions that include regulation of kinases, generation of anti-sigma factor antagonists, and regulation of circadian clocks. Here we present the crystal structure, photocycle kinetics, association properties, and spectroscopic features of a full-length LOV domain protein from Rhodobacter sphaeroides (RsLOV). RsLOV exhibits N- and C-terminal helical extensions that form an unusual helical bundle at its dimer interface with some resemblance to the helical transducer of sensory rhodopsin II. The blue light-induced conformational changes of RsLOV revealed from a comparison of light- and dark-state crystal structures support a shared signaling mechanism of LOV domain proteins that originates with the light-induced formation of a flavin-cysteinyl photoadduct. Adduct formation disrupts hydrogen bonding in the active site and propagates structural changes through the LOV domain core to the N- and C-terminal extensions. Single-residue variants in the active site and dimer interface of RsLOV alter photoadduct lifetimes and induce structural changes that perturb the oligomeric state. Size exclusion chromatography, multiangle light scattering, small-angle X-ray scattering, and cross-linking studies indicate that RsLOV dimerizes in the dark but, upon light excitation, dissociates into monomers. This light-induced switch in oligomeric state may prove to be useful for engineering molecular associations in controlled cellular settings.
Figures

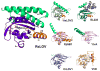
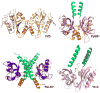


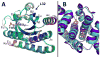

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

