Therapy development for diffuse axonal injury
- PMID: 23252624
- PMCID: PMC3627407
- DOI: 10.1089/neu.2012.2825
Therapy development for diffuse axonal injury
Abstract
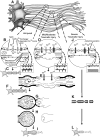
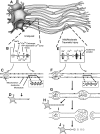
Diffuse axonal injury (DAI) remains a prominent feature of human traumatic brain injury (TBI) and a major player in its subsequent morbidity. The importance of this widespread axonal damage has been confirmed by multiple approaches including routine postmortem neuropathology as well as advanced imaging, which is now capable of detecting the signatures of traumatically induced axonal injury across a spectrum of traumatically brain-injured persons. Despite the increased interest in DAI and its overall implications for brain-injured patients, many questions remain about this component of TBI and its potential therapeutic targeting. To address these deficiencies and to identify future directions needed to fill critical gaps in our understanding of this component of TBI, the National Institute of Neurological Disorders and Stroke hosted a workshop in May 2011. This workshop sought to determine what is known regarding the pathogenesis of DAI in animal models of injury as well as in the human clinical setting. The workshop also addressed new tools to aid in the identification of this axonal injury while also identifying more rational therapeutic targets linked to DAI for continued preclinical investigation and, ultimately, clinical translation. This report encapsulates the oral and written components of this workshop addressing key features regarding the pathobiology of DAI, the biomechanics implicated in its initiating pathology, and those experimental animal modeling considerations that bear relevance to the biomechanical features of human TBI. Parallel considerations of alternate forms of DAI detection including, but not limited to, advanced neuroimaging, electrophysiological, biomarker, and neurobehavioral evaluations are included, together with recommendations for how these technologies can be better used and integrated for a more comprehensive appreciation of the pathobiology of DAI and its overall structural and functional implications. Lastly, the document closes with a thorough review of the targets linked to the pathogenesis of DAI, while also presenting a detailed report of those target-based therapies that have been used, to date, with a consideration of their overall implications for future preclinical discovery and subsequent translation to the clinic. Although all participants realize that various research gaps remained in our understanding and treatment of this complex component of TBI, this workshop refines these issues providing, for the first time, a comprehensive appreciation of what has been done and what critical needs remain unfulfilled.
Figures

References
-
- Adams J.H. Graham D.I. Murray L.S. Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann. Neurol. 1982;12:557–563. - PubMed
-
- Adams J.H. Doyle D. Graham D.I. Lawrence A.E. McLellan D.R. Diffuse axonal injury in head injuries caused by a fall. Lancet. 1984;2:1420–1422. - PubMed
-
- Adams J.H. Doyle D. Ford I. Gennarelli T.A. Graham D.I. McLellan D.R. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15:49–59. - PubMed
-
- Graham DI. Adams J.H. Gennarelli T.A. Mechanisms of non-penetrating head injury. Prog. Clin. Biol. Res. 1988;264:159–168. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

