Feasibility of frequent patient-reported outcome surveillance in patients undergoing hematopoietic cell transplantation
- PMID: 23253558
- PMCID: PMC3616493
- DOI: 10.1016/j.bbmt.2012.11.014
Feasibility of frequent patient-reported outcome surveillance in patients undergoing hematopoietic cell transplantation
Abstract
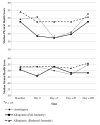
Patient-reported outcomes (PROs), including symptoms and health-related quality of life (HRQOL), provide a patient-centered description of hematopoietic cell transplantation (HCT)-related toxicity. These data characterize the patient experience after HCT and may have prognostic usefulness for long-term outcomes after HCT. We conducted a study of 32 patients after HCT (10 autologous HCT recipients, 11 full-intensity conditioning allogeneic HCT recipients, and 11 reduced-intensity conditioning allogeneic HCT recipients) to determine the feasibility of weekly electronic PRO collection from HCT until day (D) +100. We used questions from the PRO version of the Common Terminology Criteria for Adverse Events to capture symptoms, and the Patient-Reported Outcomes Measurement Information System Global Health scale to measure physical and mental HRQOL. The vast majority (94%) of patients used the electronic PRO system, with only 6% opting for paper-and-pencil only. The median weekly percentage of participants who completed the surveys was 100% in all cohorts through hospital discharge, and remained 100% for the autologous HCT and reduced-intensity allogeneic HCT cohorts through D+100. Patients were satisfied with the electronic system, giving high marks for readability, comfort, and questionnaire length. Symptom severity varied by absolute level and type of symptom across the 3 cohorts, with the full-intensity allogeneic HCT cohort exhibiting the greatest median overall symptom severity, peaking at D+7. Median physical health HRQOL scores decreased with time in the 3 cohorts, and HRQOL was generally correlated with overall symptom severity. Our results demonstrate the feasibility of frequent electronic PROs in the early post-HCT period. Future studies in larger populations to explore predictive models using frequent PRO data for outcomes, including long-term HRQOL and survival, are warranted.
Copyright © 2013 American Society for Blood and Marrow Transplantation. All rights reserved.
Figures


References
-
- Pasquini MC, Wang Z. [Accessed October 18, 2012];Current use and outcome of hematopoietic stem cell transplantation: CIBMTR summary slides. 2011 Available from: http://www.cibmtr.org.
-
- Lee SJ, Fairclough D, Parsons SK, et al. Recovery after stem-cell transplantation for hematologic diseases. J Clin Oncol. 2001;19:242–252. - PubMed
-
- Andrykowski MA, Bishop MM, Hahn EA, et al. Long-term health-related quality of life, growth, and spiritual well-being after hematopoietic stem-cell transplantation. J Clin Oncol. 2005;23:599–608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

