Long-term follow-up after gene therapy for canavan disease
- PMID: 23253610
- PMCID: PMC3794457
- DOI: 10.1126/scitranslmed.3003454
Long-term follow-up after gene therapy for canavan disease
Abstract
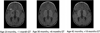
Canavan disease is a hereditary leukodystrophy caused by mutations in the aspartoacylase gene (ASPA), leading to loss of enzyme activity and increased concentrations of the substrate N-acetyl-aspartate (NAA) in the brain. Accumulation of NAA results in spongiform degeneration of white matter and severe impairment of psychomotor development. The goal of this prospective cohort study was to assess long-term safety and preliminary efficacy measures after gene therapy with an adeno-associated viral vector carrying the ASPA gene (AAV2-ASPA). Using noninvasive magnetic resonance imaging and standardized clinical rating scales, we observed Canavan disease in 28 patients, with a subset of 13 patients being treated with AAV2-ASPA. Each patient received 9 × 10(11) vector genomes via intraparenchymal delivery at six brain infusion sites. Safety data collected over a minimum 5-year follow-up period showed a lack of long-term adverse events related to the AAV2 vector. Posttreatment effects were analyzed using a generalized linear mixed model, which showed changes in predefined surrogate markers of disease progression and clinical assessment subscores. AAV2-ASPA gene therapy resulted in a decrease in elevated NAA in the brain and slowed progression of brain atrophy, with some improvement in seizure frequency and with stabilization of overall clinical status.
Figures



References
-
- Hagenfeldt L, Bollgren I, Venizelos N. N-acetylaspartic aciduria due to aspartoacylase deficiency—A new aetiology of childhood leukodystrophy. J. Inher. Metab. Dis. 1987;10:135–141. - PubMed
-
- Matalon R, Michals K, Sebesta D, Deanching M, Gashkoff P, Casanova J. Aspartoacylase deficiency and N-acetylaspartic aciduria in patients with Canavan disease. Am. J. Med. Genet. 1988;29:463–471. - PubMed
-
- Kaul R, Gao GP, Balamurugan K, Matalon R. Cloning of the human aspartoacylase cDNA and a common missense mutation in Canavan disease. Nat. Genet. 1993;5:118–123. - PubMed
-
- Kaul R, Casanova J, Johnson AB, Tang P, Matalon R. Purification characterization and localization of aspartoacylase from bovine brain. J. Neurochem. 1991;56:129–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

