Efficacy of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder previously treated with amphetamines: analyses from a randomized, double-blind, multicenter, placebo-controlled titration study
- PMID: 23254273
- PMCID: PMC3554536
- DOI: 10.1186/2050-6511-13-18
Efficacy of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder previously treated with amphetamines: analyses from a randomized, double-blind, multicenter, placebo-controlled titration study
Abstract
Background: To examine the efficacy of lisdexamfetamine dimesylate (LDX) in adults with attention-deficit/hyperactivity disorder (ADHD) who remained symptomatic (ADHD Rating Scale IV [ADHD-RS-IV] total score >18) on amphetamine (AMPH) therapy (mixed AMPH salts and/or d-AMPH formulations) prior to enrollment in a 4-week placebo-controlled LDX trial vs the overall study population. In these post hoc analyses from a multicenter, randomized, double-blind, forced-dose titration study, clinical efficacy of LDX (30-70 mg/d) in adults with ADHD receiving AMPH treatment at screening vs the overall study population was evaluated. ADHD symptoms were assessed using the ADHD-RS-IV with adult prompts at screening, baseline (after prior treatment washout), and endpoint. Safety assessments included treatment-emergent adverse events (TEAEs), vital signs, laboratory findings, and electrocardiogram.
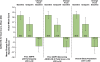
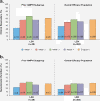
Results: Of 414 participants (62, placebo; 352, LDX) included in the overall study population, 41 were receiving AMPH therapy at screening (2, placebo; 39, LDX); mean AMPH dose was 35.0 and 34.1 mg/d for participants in placebo and all LDX groups, respectively. Of the 41 participants, 36 remained symptomatic (ADHD-RS-IV >18) at screening despite receiving AMPH. For the 36 participants in the placebo (n = 2) and LDX (n = 34) groups, respectively, at endpoint, mean change from screening ADHD-RS-IV total scores were -5.5 and -14.8 and from baseline scores were -13.5 and -17.8. For the overall study population, endpoint mean change from baseline ADHD-RS-IV total scores were -7.8 for placebo and -17.5 for LDX. In the prior AMPH subgroup, 2/2 (100.0%) in the placebo group and 22/39 (56.4%) participants in the LDX (all doses) group reported any TEAE. Events that occurred in ≥5% for LDX were dry mouth (5/39; 12.8%), headache (5/39; 12.8%), fatigue (3/39; 7.7%), insomnia (3/39; 7.7%), decreased appetite (2/39; 5.1%), and nausea (2/39; 5.1%). None of these events occurred in the 2 placebo patients with prior AMPH use.
Conclusion: In these post hoc analyses, adults with significant baseline ADHD symptoms despite adequate AMPH treatment dose showed similar improvements in ADHD symptoms with LDX treatment as the overall study population. Prospective studies are needed to confirm these findings. The safety profile of LDX in the overall study population was consistent with long-acting psychostimulant use.
Trial registry: Study to Assess the Safety and Efficacy of NRP104 in Adults With Attention-Deficit Hyperactivity Disorder (ADHD). Clinicaltrials.gov Identifier: NCT00334880.
Figures
References
-
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K. et al.The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716. doi: 10.1176/appi.ajp.163.4.716. - DOI - PMC - PubMed
-
- Pliszka SR, Crismon ML, Hughes CW, Conners CK, Emslie GJ, Jensen PS, McCracken JT, Swanson JM, Lopez M. The Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention-Deficit/Hyperactivity Disorder. The Texas Children's Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45(6):642. doi: 10.1097/01.chi.0000215326.51175.eb. - DOI - PubMed
-
- Canadian ADHD Resource Alliance. Canadian ADHD practice guidelines. CAP-guidelines 3rd edition. http://www.caddra.ca/cms4/pdfs/caddraGuidelines2011.pdf. Accessed September 13, 2012.
-
- Nutt DJ, Fone K, Asherson P, Bramble D, Hill P, Matthews K, Morris KA, Santosh P, Sonuga-Barke E, Taylor E. et al.Evidence-based guidelines for management of attention-deficit/hyperactivity disorder in adolescents in transition to adult services and in adults: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2007;21(1):10. - PubMed
-
- Arnold LE. Methylphenidate vs. amphetamine: comparative review. J Atten Disord. 2000;3(4):200. doi: 10.1177/108705470000300403. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

