Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion
- PMID: 23254287
- PMCID: PMC3549717
- DOI: 10.1084/jem.20121172
Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion
Abstract
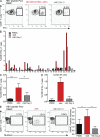
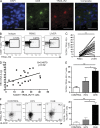
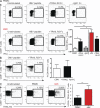
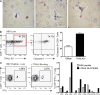
Antiviral T cell responses in hepatotropic viral infections such as hepatitis B virus (HBV) are profoundly diminished and prone to apoptotic deletion. In this study, we investigate whether the large population of activated NK cells in the human liver contributes to this process. We show that in vitro removal of NK cells augments circulating CD8(+) T cell responses directed against HBV, but not against well-controlled viruses, in patients with chronic hepatitis B (CHB). We find that NK cells can rapidly eliminate HBV-specific T cells in a contact-dependent manner. CD8(+) T cells in the liver microcirculation are visualized making intimate contact with NK cells, which are the main intrahepatic lymphocytes expressing TNF-related apoptosis-inducing ligand (TRAIL) in CHB. High-level expression of the TRAIL death receptor TRAIL-R2 is found to be a hallmark of T cells exposed to the milieu of the HBV-infected liver in patients with active disease. Up-regulation of TRAIL-R2 renders T cells susceptible to caspase-8-mediated apoptosis, from which they can be partially rescued by blockade of this death receptor pathway. Our findings demonstrate that NK cells can negatively regulate antiviral immunity in chronic HBV infection and illustrate a novel mechanism of T cell tolerance in the human liver.
Figures




References
-
- Andrews D.M., Estcourt M.J., Andoniou C.E., Wikstrom M.E., Khong A., Voigt V., Fleming P., Tabarias H., Hill G.R., van der Most R.G., et al. 2010. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J. Exp. Med. 207:1333–1343 10.1084/jem.20091193 - DOI - PMC - PubMed
-
- Cerboni C., Zingoni A., Cippitelli M., Piccoli M., Frati L., Santoni A. 2007. Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK- cell lysis. Blood. 110:606–615 10.1182/blood-2006-10-052720 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

