Senescence-associated secretory phenotype favors the emergence of cancer stem-like cells
- PMID: 23254289
- PMCID: PMC3542619
- DOI: 10.1038/cddis.2012.183
Senescence-associated secretory phenotype favors the emergence of cancer stem-like cells
Abstract
The molecular mechanisms underlying cancer resistance remain elusive. One possible explanation is that cancer stem cells (CSCs) elude drug treatment, emerge and reproduce a tumor. Using multiple myeloma as a paradigm, we showed that cancer stem-like cells (CSLCs) appear after genotoxic stress because of their intrinsic properties. However, these properties do not drive the emergence of the CSLCs. Following genotoxic stress, remaining DNA damages lead to a senescence-associated secretory phenotype (SASP). Senescent cells, which are the non-CSLCs, secrete chemokines contributing to the emergence, maintenance and migration of CSLCs. Downregulation of checkpoint protein 2, a key player of SASP, significantly reduced the emergence of CSLCs. Our results unravel a novel molecular mechanism by which SASP might promote malignancy, underlining the dual role of senescence in tumorigenesis. This mechanism, based on mutual cooperation among tumor cells, illustrates how cancer may relapse; its targeting could represent new therapeutic opportunities.
Figures
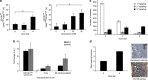
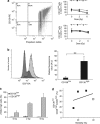


References
-
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells – perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. - PubMed
-
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

