Lucidone suppresses hepatitis C virus replication by Nrf2-mediated heme oxygenase-1 induction
- PMID: 23254429
- PMCID: PMC3591928
- DOI: 10.1128/AAC.02053-12
Lucidone suppresses hepatitis C virus replication by Nrf2-mediated heme oxygenase-1 induction
Abstract
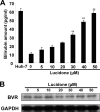
Upon screening of plant-derived natural products against hepatitis C virus (HCV) in the replicon system, we demonstrate that lucidone, a phytocompound, isolated from the fruits of Lindera erythrocarpa Makino, significantly suppressed HCV RNA levels with 50% effective concentrations of 15 ± 0.5 μM and 20 ± 1.1 μM in HCV replicon and JFH-1 infectious assays, respectively. There was no significant cytotoxicity observed at high concentrations, with a 50% cytotoxic concentration of 620 ± 5 μM. In addition, lucidone significantly induced heme oxygenase-1 (HO-1) production and led to the increase of its product biliverdin for inducing antiviral interferon response and inhibiting HCV NS3/4A protease activity. Conversely, the anti-HCV activity of lucidone was abrogated by blocking HO-1 activity or silencing gene expression of HO-1 or NF-E2-related factor 2 (Nrf2) in the presence of lucidone, indicating that the anti-HCV action of lucidone was due to the stimulation of Nrf-2-mediated HO-1 expression. Moreover, the combination of lucidone and alpha interferon, the protease inhibitor telaprevir, the NS5A inhibitor BMS-790052, or the NS5B polymerase inhibitor PSI-7977, synergistically suppressed HCV RNA replication. These findings suggest that lucidone could be a potential lead or supplement for the development of new anti-HCV agent in the future.
Figures








Similar articles
-
Sulforaphane Suppresses Hepatitis C Virus Replication by Up-Regulating Heme Oxygenase-1 Expression through PI3K/Nrf2 Pathway.PLoS One. 2016 Mar 29;11(3):e0152236. doi: 10.1371/journal.pone.0152236. eCollection 2016. PLoS One. 2016. PMID: 27023634 Free PMC article.
-
Celastrol inhibits hepatitis C virus replication by upregulating heme oxygenase-1 via the JNK MAPK/Nrf2 pathway in human hepatoma cells.Antiviral Res. 2017 Oct;146:191-200. doi: 10.1016/j.antiviral.2017.09.010. Epub 2017 Sep 19. Antiviral Res. 2017. PMID: 28935193 Free PMC article.
-
Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cells.Br J Pharmacol. 2014 Jan;171(1):237-52. doi: 10.1111/bph.12440. Br J Pharmacol. 2014. PMID: 24117426 Free PMC article.
-
TMC-435, an NS3/4A protease inhibitor for the treatment of HCV infection.Curr Opin Investig Drugs. 2009 Aug;10(8):871-81. Curr Opin Investig Drugs. 2009. PMID: 19649931 Review.
-
Macrocyclic Hepatitis C Virus NS3/4A Protease Inhibitors: An Overview of Medicinal Chemistry.Curr Med Chem. 2016;23(29):3404-3447. doi: 10.2174/0929867323666160510122525. Curr Med Chem. 2016. PMID: 27160539 Review.
Cited by
-
Lucidone suppresses dengue viral replication through the induction of heme oxygenase-1.Virulence. 2018 Jan 1;9(1):588-603. doi: 10.1080/21505594.2017.1421893. Virulence. 2018. PMID: 29338543 Free PMC article.
-
Sulforaphane Suppresses Hepatitis C Virus Replication by Up-Regulating Heme Oxygenase-1 Expression through PI3K/Nrf2 Pathway.PLoS One. 2016 Mar 29;11(3):e0152236. doi: 10.1371/journal.pone.0152236. eCollection 2016. PLoS One. 2016. PMID: 27023634 Free PMC article.
-
Human heme oxygenase 1 is a potential host cell factor against dengue virus replication.Sci Rep. 2016 Aug 24;6:32176. doi: 10.1038/srep32176. Sci Rep. 2016. PMID: 27553177 Free PMC article.
-
Therapeutic Potential of Lindera obtusiloba: Focus on Antioxidative and Pharmacological Properties.Plants (Basel). 2020 Dec 13;9(12):1765. doi: 10.3390/plants9121765. Plants (Basel). 2020. PMID: 33322185 Free PMC article. Review.
-
Comparative protein profiling of B16 mouse melanoma cells susceptible and non-susceptible to alphavirus infection: Effect of the tumor microenvironment.Cancer Biol Ther. 2016 Oct 2;17(10):1035-1050. doi: 10.1080/15384047.2016.1219813. Epub 2016 Sep 16. Cancer Biol Ther. 2016. PMID: 27636533 Free PMC article.
References
-
- Giannini C, Brechot C. 2003. Hepatitis C virus biology. Cell Death Differ. 10(Suppl 1):S27–S38 - PubMed
-
- Hayashi N, Takehara T. 2006. Antiviral therapy for chronic hepatitis C: past, present, and future. J. Gastroenterol. 41:17–27 - PubMed
-
- Schaefer M, Mauss S. 2008. Hepatitis C treatment in patients with drug addiction: clinical management of interferon-alpha-associated psychiatric side effects. Curr. Drug Abuse Rev. 1:177–187 - PubMed
-
- Susser S, Welsch C, Wang Y, Zettler M, Domingues FS, Karey U, Hughes E, Ralston R, Tong X, Herrmann E, Zeuzem S, Sarrazin C. 2009. Characterization of resistance to the protease inhibitor boceprevir in hepatitis C virus-infected patients. Hepatology 50:1709–1718 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

