Randomized, open-label trial of primaquine against vivax malaria relapse in Indonesia
- PMID: 23254437
- PMCID: PMC3591862
- DOI: 10.1128/AAC.01879-12
Randomized, open-label trial of primaquine against vivax malaria relapse in Indonesia
Abstract
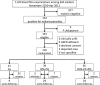
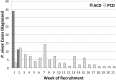
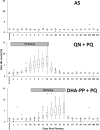
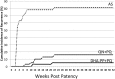
Radical cure of Plasmodium vivax infection applies blood schizontocidal therapy against the acute attack and hypnozoitocidal therapy against later relapse. Chloroquine and primaquine have been used for 60 years in this manner. Resistance to chloroquine by the parasite now requires partnering other blood schizontocides with primaquine. However, the safety and efficacy of primaquine against relapse when combined with other drugs have not been demonstrated. This randomized, open-label, and relapse-controlled trial estimated the efficacy of primaquine against relapse when administered with quinine or dihydroartemisinin-piperaquine for treatment of the acute infection. Among 650 soldiers who had returned to their malaria-free base in Java, Indonesia, after 12 months in malarious Papua, Indonesia, 143 with acute P. vivax malaria were eligible for study. One hundred sixteen enrolled subjects were randomized to these treatments: artesunate (200-mg dose followed by 100 mg/day for 6 days), quinine (1.8 g/day for 7 days) plus concurrent primaquine (30 mg/day for 14 days), or dihydroartemisinin (120 mg) plus piperaquine (960 mg) daily for 3 days followed 25 days later by primaquine (30 mg/day for 14 days). Follow-up was for 12 months. One hundred thirteen subjects were analyzable. Relapse occurred in 32 of 41 (78%) subjects administered artesunate alone (2.71 attacks/person-year), 7 of 36 (19%) administered quinine plus primaquine (0.23 attack/person-year), and 2 of 36 (6%) administered dihydroartemisinin-piperaquine plus primaquine (0.06 attack/person-year). The efficacy of primaquine against relapse was 92% (95% confidence interval [CI] = 81% to 96%) for quinine plus primaquine and 98% (95% CI = 91% to 99%) for dihydroartemisinin-piperaquine plus primaquine. Antirelapse therapy with primaquine begun a month after treatment of the acute attack with dihydroartemisinin-piperaquine proved safe and highly efficacious against relapse by P. vivax acquired in Papua, Indonesia.
Figures

References
-
- Mendis K, Sina BJ, Marchesini P, Carter R. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64(1–2 Suppl):97–106 - PubMed
-
- Baird JK. 2007. Neglect of Plasmodium vivax malaria. Trends Parasitol. 23:533–539 - PubMed
-
- Guerra CA, Howes RE, Patil PA, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IR, Baird JK, Snow RW, Hay SI. 2010. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl. Trop. Dis. 4:e774 doi:10.1371/journal.pntd.0000774 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

