Multivalent glycoconjugates as anti-pathogenic agents
- PMID: 23254759
- PMCID: PMC4399576
- DOI: 10.1039/c2cs35408j
Multivalent glycoconjugates as anti-pathogenic agents
Abstract
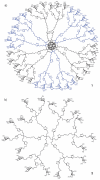
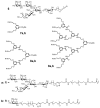
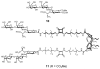
Multivalency plays a major role in biological processes and particularly in the relationship between pathogenic microorganisms and their host that involves protein-glycan recognition. These interactions occur during the first steps of infection, for specific recognition between host and bacteria, but also at different stages of the immune response. The search for high-affinity ligands for studying such interactions involves the combination of carbohydrate head groups with different scaffolds and linkers generating multivalent glycocompounds with controlled spatial and topology parameters. By interfering with pathogen adhesion, such glycocompounds including glycopolymers, glycoclusters, glycodendrimers and glyconanoparticles have the potential to improve or replace antibiotic treatments that are now subverted by resistance. Multivalent glycoconjugates have also been used for stimulating the innate and adaptive immune systems, for example with carbohydrate-based vaccines. Bacteria present on their surfaces natural multivalent glycoconjugates such as lipopolysaccharides and S-layers that can also be exploited or targeted in anti-infectious strategies.
Figures


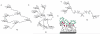
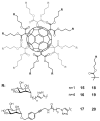
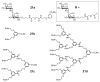
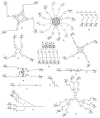


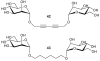

References
-
- Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. Essentials of Glycobiology. 2nd edn Cold Spring Harbour; New York: 2009. - PubMed
-
- Gabius HJ, Andre S, Jimenez-Barbero J, Romero A, Solis D. Trends Biochem. Sci. 2012;36:298–313. - PubMed
-
- Lis H, Sharon N. Chem. Rev. 1998;98:637–674. - PubMed
-
- Kiessling LL, Gestwicki JE, Strong LE. Curr. Opin. Chem. Biol. 2000;4:696–703. - PubMed
-
- Lee YC, Lee RT. Acc. Chem. Res. 1995;28:321–327.

