Role of cranial and spinal virtual and augmented reality simulation using immersive touch modules in neurosurgical training
- PMID: 23254799
- PMCID: PMC3676942
- DOI: 10.1227/NEU.0b013e3182753093
Role of cranial and spinal virtual and augmented reality simulation using immersive touch modules in neurosurgical training
Erratum in
- Neurosurgery. 2013 Nov;73(5):E913. Tobin, Mathew [corrected to Tobin, Matthew]
Abstract
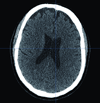
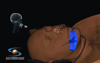
Recent studies have shown that mental script-based rehearsal and simulation-based training improve the transfer of surgical skills in various medical disciplines. Despite significant advances in technology and intraoperative techniques over the last several decades, surgical skills training on neurosurgical operations still carries significant risk of serious morbidity or mortality. Potentially avoidable technical errors are well recognized as contributing to poor surgical outcome. Surgical education is undergoing overwhelming change, as a result of the reduction of work hours and current trends focusing on patient safety and linking reimbursement with clinical outcomes. Thus, there is a need for adjunctive means for neurosurgical training, which is a recent advancement in simulation technology. ImmersiveTouch is an augmented reality system that integrates a haptic device and a high-resolution stereoscopic display. This simulation platform uses multiple sensory modalities, re-creating many of the environmental cues experienced during an actual procedure. Modules available include ventriculostomy, bone drilling, percutaneous trigeminal rhizotomy, and simulated spinal modules such as pedicle screw placement, vertebroplasty, and lumbar puncture. We present our experience with the development of such augmented reality neurosurgical modules and the feedback from neurosurgical residents.
Figures

















References
-
- Aggarwal R, Black SA, Hance JR, Darzi A, Cheshire NJ. Virtual reality simulation training can improve inexperienced surgeons' endovascular skills. Eur J Vasc Endovasc Surg. 2006 Jun;31(6):588–593. - PubMed
-
- Fried MP, Sadoughi B, Gibber MJ, et al. From virtual reality to the operating room: the endoscopic sinus surgery simulator experiment. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2010 Feb;142(2):202–207. - PubMed
-
- Grantcharov TP. Is virtual reality simulation an effective training method in surgery? Nature clinical practice. Gastroenterology & hepatology. 2008 May;5(5):232–233. - PubMed
-
- Palter VN, Graafland M, Schijven MP, Grantcharov TP. Designing a proficiency-based, content validated virtual reality curriculum for laparoscopic colorectal surgery: a Delphi approach. Surgery. 2012 Mar;151(3):391–397. - PubMed
-
- Arabi Y, Memish ZA, Balkhy HH, et al. Ventriculostomy-associated infections: incidence and risk factors. American journal of infection control. 2005 Apr;33(3):137–143. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

