Population pharmacokinetics of phenobarbital in infants with neonatal encephalopathy treated with therapeutic hypothermia
- PMID: 23254984
- PMCID: PMC3717607
- DOI: 10.1097/PCC.0b013e31825bbbc2
Population pharmacokinetics of phenobarbital in infants with neonatal encephalopathy treated with therapeutic hypothermia
Abstract
Objective: Phenobarbital is the first-line treatment for neonatal seizures. Many neonates with hypoxic ischemic encephalopathy are treated with therapeutic hypothermia, and about 40% have clinical seizures. Little is known about the pharmacokinetics of phenobarbital in infants with hypoxic ischemic encephalopathy who undergo therapeutic hypothermia. The objective of this study was to determine the effect of therapeutic hypothermia on phenobarbital pharmacokinetics, taking into account maturational changes.
Setting: Level 3 neonatal ICU.
Patients: Infants with hypoxic ischemic encephalopathy and suspected seizures, all treated with phenobarbital. Some of these infants also received treatment with therapeutic hypothermia.
Interventions: None.
Design: A retrospective cohort study of 39 infants with hypoxic ischemic encephalopathy treated with phenobarbital (20 were treated with therapeutic hypothermia and 19 were not).
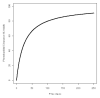
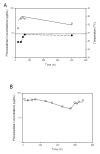
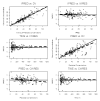
Measurements and main results: Data on phenobarbital plasma concentrations were collected in 39 subjects with hypoxic ischemic encephalopathy with or without therapeutic hypothermia. Using nonlinear mixed-effects modeling, population pharmacokinetics of phenobarbital were developed with a total of 164 plasma concentrations. A one-compartment model best described the pharmacokinetics. The clearance of phenobarbital was linearly related to body weight and matured with increasing age with a maturation half-life of 22.1 days. Therapeutic hypothermia did not influence the pharmacokinetic parameters of phenobarbital.
Conclusions: Therapeutic hypothermia does not influence the clearance of phenobarbital after accounting for weight and age. Standard phenobarbital dosing is appropriate for the initial treatment of seizures in neonates with hypoxic ischemic encephalopathy treated with therapeutic hypothermia.
Figures

Comment in
-
Drug dosing during hypothermia: to adjust, or not to adjust, that is the question.Pediatr Crit Care Med. 2013 Feb;14(2):228-9. doi: 10.1097/PCC.0b013e31826775cd. Pediatr Crit Care Med. 2013. PMID: 23388572 Free PMC article. No abstract available.
References
-
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. - PubMed
-
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, et al. Whole body hypothermia for neonates with hypoxic-ischemic encephalopathy. New England Journal of Medicine. 2005;353:1574–1584. - PubMed
-
- Wyatt JS, Gluckman PD, Liu PY, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–921. - PubMed
-
- Bartha AI, Shen J, Katz KH, Mischel RE, Yap KR, Ivacko JA, Andrews EM, Ferriero DM, Ment LR, FS. S. Neonatal Seizures: Multicenter Variability in Current Treatment Practices. Pediatric Neurology. 2007;37:85–90. - PubMed
-
- Blume HK, Garrison MM, Christakis DA. Neonatal seizures: treatment and treatment variability in 31 United States pediatric hospitals. Journal of Child Neurology. 2009;24(2):148–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

