Delineation of the clinical, molecular and cellular aspects of novel JAM3 mutations underlying the autosomal recessive hemorrhagic destruction of the brain, subependymal calcification, and congenital cataracts
- PMID: 23255084
- PMCID: PMC3951164
- DOI: 10.1002/humu.22263
Delineation of the clinical, molecular and cellular aspects of novel JAM3 mutations underlying the autosomal recessive hemorrhagic destruction of the brain, subependymal calcification, and congenital cataracts
Abstract
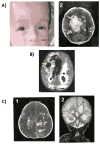
We have recently shown that the hemorrhagic destruction of the brain, subependymal, calcification, and congenital cataracts is caused by biallelic mutations in the gene encoding junctional adhesion molecule 3 (JAM3) protein. Affected members from three new families underwent detailed clinical examination including imaging of the brain. Affected individuals presented with a distinctive phenotype comprising hemorrhagic destruction of the brain, subependymal calcification, and congenital cataracts. All patients had a catastrophic clinical course resulting in death. Sequencing the coding exons of JAM3 revealed three novel homozygous mutations: c.2T>G (p.M1R), c.346G>A (p.E116K), and c.656G>A (p.C219Y). The p.M1R mutation affects the start codon and therefore is predicted to impair protein synthesis. Cellular studies showed that the p.C219Y mutation resulted in a significant retention of the mutated protein in the endoplasmic reticulum, suggesting a trafficking defect. The p.E116K mutant traffics normally to the plasma membrane as the wild-type and may have lost its function due to the lack of interaction with an interacting partner. Our data further support the importance of JAM3 in the development and function of the vascular system and the brain.
© 2012 Wiley Periodicals, Inc.
Conflict of interest statement
All the authors declare no conflict of interest.
Figures

References
-
- Abdel-Salam GM, Zaki MS, Saleem SN, Gaber KR. Microcephaly, malformation of brain development and intracranial calcification in sibs: pseudo-TORCH or a new syndrome. Am J Med Genet. 2008;146A:2929–2936. - PubMed
-
- Aridor M. Visiting the ER: the endoplasmic reticulum as a target for therapeutics in traffic related diseases. Adv Drug Deliv Rev. 2007;59:759–781. - PubMed
-
- Arrate MP, Rodriguez JM, Tran TT, Brock TA, Cunningham SA. Cloning of human junctional adhesion molecule 3 (JAM3) and its identification as the JAM2 counter-receptor. J Biol Chem. 2001;276:45826–45832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

