Regulation of miR106b cluster through the RB pathway: mechanism and functional targets
- PMID: 23255112
- PMCID: PMC3570523
- DOI: 10.4161/cc.23029
Regulation of miR106b cluster through the RB pathway: mechanism and functional targets
Abstract
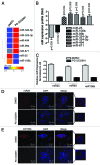
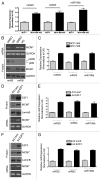
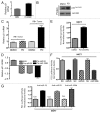
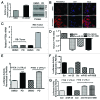
The RB pathway plays a critical role in proliferation control that is commonly subverted in tumor development. However, restoration of RB pathway function can be elicited in many tumor cells by the inhibition of CDK4/6 activity that leads to dephosphorylation of RB and subsequent repression of E2F-mediated transcription. In this context, active RB/E2F complexes inhibit the expression of a critical program of coding genes that promote cell cycle progression. However, the non-coding RNA target genes downstream from RB that could be relevant for tumor biology remain obscure. Here, miRNA gene expression profiling identified the miR106b cluster as being efficiently repressed with CDK4/6 inhibition in an E2F and RB-dependent manner. Importantly, the miR106B-cluster is intragenic of MCM7, and through a series of functional studies, the basis of MCM7 regulation and concordant expression of the miRNA species within the 106b cluster was determined. Importantly, RB-mediated repression of the 106b cluster enhances the transcript levels of p21Cip1 and PTEN. These data provide a mechanistic basis for cross-talk between the RB pathway and p21 and PTEN through the regulation of the MCM7/miR106b locus.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
