Combination of a selective activator of the glucocorticoid receptor Compound A with a proteasome inhibitor as a novel strategy for chemotherapy of hematologic malignancies
- PMID: 23255118
- PMCID: PMC3570502
- DOI: 10.4161/cc.23048
Combination of a selective activator of the glucocorticoid receptor Compound A with a proteasome inhibitor as a novel strategy for chemotherapy of hematologic malignancies
Abstract
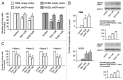
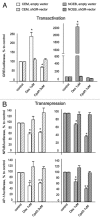
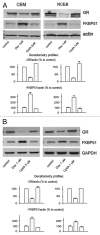
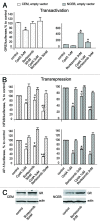
Glucocorticoids are widely used for the treatment of hematological malignancies; however, their chronic use results in numerous metabolic side effects. Thus, the development of selective glucocorticoid receptor (GR) activators (SEGRA) with improved therapeutic index is important. GR regulates gene expression via (1) transactivation that requires GR homodimer binding to gene promoters and is linked to side effects and (2) transrepression-mediated via negative GR interaction with other transcription factors. Novel GR modulator Compound A (CpdA) prevents GR dimerization, retains glucocorticoid anti-inflammatory activity and has fewer side effects compared with glucocorticoids in vivo. Here we tested CpdA anticancer activity in human T- and B-lymphoma and multiple myeloma cells expressing GR and their counterparts with silenced GR. We found that CpdA in GR-dependent manner strongly inhibited growth and viability of human T-, B-lymphoma and multiple myeloma cells. Furthermore, primary leukemia cell cultures from T-ALL patients appeared to be equally sensitive to glucocorticoid dexamethasone and CpdA. It is known that GR expression is controlled by proteasome. We showed that pretreatment of lymphoma CEM and NCEB cells with proteasome-inhibitor Bortezomib resulted in GR accumulation and enhanced ligand properties of CpdA, shifting GR activity toward transrepression evaluated by inhibition of NFкB and AP-1 transcription factors. We also revealed remarkable GR-dependent cooperation between CpdA and Bortezomib in suppressing growth and survival of T- and B-lymphoma and multiple myeloma MM.1S cells. Overall, our data provide the rationale for novel GR-based therapy for hematological malignancies based on combination of SEGRA with proteasome inhibitors.
Figures


Similar articles
-
Antitumor effect of non-steroid glucocorticoid receptor ligand CpdA on leukemia cell lines CEM and K562.Biochemistry (Mosc). 2011 Nov;76(11):1242-52. doi: 10.1134/S000629791111006X. Biochemistry (Mosc). 2011. PMID: 22117551
-
Differential targeting of androgen and glucocorticoid receptors induces ER stress and apoptosis in prostate cancer cells: a novel therapeutic modality.Cell Cycle. 2012 Jan 15;11(2):395-406. doi: 10.4161/cc.11.2.18945. Epub 2012 Jan 15. Cell Cycle. 2012. PMID: 22223138 Free PMC article.
-
Selective modulation of the glucocorticoid receptor can distinguish between transrepression of NF-κB and AP-1.Cell Mol Life Sci. 2014 Jan;71(1):143-63. doi: 10.1007/s00018-013-1367-4. Epub 2013 Jun 20. Cell Mol Life Sci. 2014. PMID: 23784308 Free PMC article.
-
Discovery of Compound A--a selective activator of the glucocorticoid receptor with anti-inflammatory and anti-cancer activity.Oncotarget. 2015 Oct 13;6(31):30730-44. doi: 10.18632/oncotarget.5078. Oncotarget. 2015. PMID: 26436695 Free PMC article. Review.
-
The proteasome: a novel target for anticancer therapy.Clin Transl Oncol. 2006 May;8(5):313-7. doi: 10.1007/s12094-006-0176-8. Clin Transl Oncol. 2006. PMID: 16760005 Review.
Cited by
-
Synthesis and Anti-Cancer Activity of the Novel Selective Glucocorticoid Receptor Agonists of the Phenylethanolamine Series.Int J Mol Sci. 2024 Aug 15;25(16):8904. doi: 10.3390/ijms25168904. Int J Mol Sci. 2024. PMID: 39201590 Free PMC article.
-
Effect of the plant derivative Compound A on the production of corticosteroid-resistant chemokines in airway smooth muscle cells.Am J Respir Cell Mol Biol. 2015 Nov;53(5):728-37. doi: 10.1165/rcmb.2014-0477OC. Am J Respir Cell Mol Biol. 2015. PMID: 25897650 Free PMC article.
-
Effect of combining glucocorticoids with Compound A on glucocorticoid receptor responsiveness in lymphoid malignancies.PLoS One. 2018 May 8;13(5):e0197000. doi: 10.1371/journal.pone.0197000. eCollection 2018. PLoS One. 2018. PMID: 29738549 Free PMC article.
-
Rapamycin Modulates Glucocorticoid Receptor Function, Blocks Atrophogene REDD1, and Protects Skin from Steroid Atrophy.J Invest Dermatol. 2018 Sep;138(9):1935-1944. doi: 10.1016/j.jid.2018.02.045. Epub 2018 Mar 27. J Invest Dermatol. 2018. PMID: 29596905 Free PMC article.
-
Synephrine and Its Derivative Compound A: Common and Specific Biological Effects.Int J Mol Sci. 2023 Dec 15;24(24):17537. doi: 10.3390/ijms242417537. Int J Mol Sci. 2023. PMID: 38139366 Free PMC article. Review.
References
-
- Rosenthal MC, Saunders RH, Schwartz LI, Zannos L, Perez Santiago E, Dameshek W. The use of adrenocorticotropic hormone and cortisone in the treatment of leukemia and leukosarcoma. Blood. 1951;6:804–23. - PubMed
-
- Greenstein S, Krett NL, Kurosawa Y, Ma C, Chauhan D, Hideshima T, et al. Characterization of the MM.1 human multiple myeloma (MM) cell lines: a model system to elucidate the characteristics, behavior, and signaling of steroid-sensitive and -resistant MM cells. Exp Hematol. 2003;31:271–82. doi: 10.1016/S0301-472X(03)00023-7. - DOI - PubMed
-
- Moreau P, Avet-Loiseau H, Facon T, Attal M, Tiab M, Hulin C, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118:5752–8, quiz 5982. doi: 10.1182/blood-2011-05-355081. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
