Human IGF1 extends lifespan and enhances resistance to Plasmodium falciparum infection in the malaria vector Anopheles stephensi
- PMID: 23255191
- PMCID: PMC3597202
- DOI: 10.1242/jeb.078873
Human IGF1 extends lifespan and enhances resistance to Plasmodium falciparum infection in the malaria vector Anopheles stephensi
Abstract
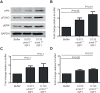
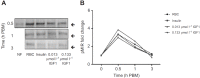
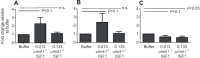
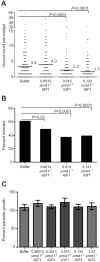
The highly conserved insulin/insulin-like growth factor (IGF) signaling (IIS) pathway regulates metabolism, development, lifespan and immunity across a wide range of organisms. Previous studies have shown that human insulin ingested in the blood meal can activate mosquito IIS, resulting in attenuated lifespan and increased malaria parasite infection. Because human IGF1 is present at higher concentrations in blood than insulin and is functionally linked with lifespan and immune processes, we predicted that human IGF1 ingested in a blood meal would affect lifespan and malaria parasite infection in the mosquito Anopheles stephensi. Here we demonstrate that physiological levels of ingested IGF1, like insulin, can persist intact in the blood-filled midgut for up to 30 h and disseminate into the mosquito body, and that both peptides activate IIS in mosquito cells and midgut. At these same levels, ingested IGF1 alone extended average mosquito lifespan by 23% compared with controls and, more significantly, when ingested in infected blood meals, reduced the prevalence of Plasmodium falciparum-infected mosquitoes by >20% and parasite load by 35-50% compared with controls. Thus, the effects of ingested IGF1 on mosquito lifespan and immunity are opposite to those of ingested insulin. These results offer the first evidence that insect cells can functionally discriminate between mammalian insulin and IGF1. Further, in light of previous success in genetically targeting IIS to alter mosquito lifespan and malaria parasite transmission, this study indicates that a more complete understanding of the IIS-activating ligands in blood can be used to optimize transgenic strategies for malaria control.
Figures


Similar articles
-
Human IGF1 regulates midgut oxidative stress and epithelial homeostasis to balance lifespan and Plasmodium falciparum resistance in Anopheles stephensi.PLoS Pathog. 2014 Jun 26;10(6):e1004231. doi: 10.1371/journal.ppat.1004231. eCollection 2014 Jun. PLoS Pathog. 2014. PMID: 24968248 Free PMC article.
-
Inhibition of JNK signaling in the Asian malaria vector Anopheles stephensi extends mosquito longevity and improves resistance to Plasmodium falciparum infection.PLoS Pathog. 2018 Nov 29;14(11):e1007418. doi: 10.1371/journal.ppat.1007418. eCollection 2018 Nov. PLoS Pathog. 2018. PMID: 30496310 Free PMC article.
-
Overexpression of phosphatase and tensin homolog improves fitness and decreases Plasmodium falciparum development in Anopheles stephensi.Microbes Infect. 2013 Nov;15(12):775-87. doi: 10.1016/j.micinf.2013.05.006. Epub 2013 Jun 15. Microbes Infect. 2013. PMID: 23774695 Free PMC article.
-
The development of malaria parasites in the mosquito midgut.Cell Microbiol. 2016 Jul;18(7):905-18. doi: 10.1111/cmi.12604. Epub 2016 May 24. Cell Microbiol. 2016. PMID: 27111866 Free PMC article. Review.
-
Interrupting malaria transmission by genetic manipulation of anopheline mosquitoes.J Vector Borne Dis. 2003 Sep-Dec;40(3-4):73-7. J Vector Borne Dis. 2003. PMID: 15119075 Review.
Cited by
-
The effects of ingested mammalian blood factors on vector arthropod immunity and physiology.Microbes Infect. 2013 Mar;15(3):243-54. doi: 10.1016/j.micinf.2013.01.003. Epub 2013 Jan 28. Microbes Infect. 2013. PMID: 23370408 Free PMC article. Review.
-
The effects of ingestion of hormonal host factors on the longevity and insecticide resistance phenotype of the major malaria vector Anopheles arabiensis (Diptera: Culicidae).PLoS One. 2017 Jul 11;12(7):e0180909. doi: 10.1371/journal.pone.0180909. eCollection 2017. PLoS One. 2017. PMID: 28700639 Free PMC article.
-
Non-immune Traits Triggered by Blood Intake Impact Vectorial Competence.Front Physiol. 2021 Mar 2;12:638033. doi: 10.3389/fphys.2021.638033. eCollection 2021. Front Physiol. 2021. PMID: 33737885 Free PMC article. Review.
-
The Ras/ERK signaling pathway couples antimicrobial peptides to mediate resistance to dengue virus in Aedes mosquitoes.PLoS Negl Trop Dis. 2020 Aug 31;14(8):e0008660. doi: 10.1371/journal.pntd.0008660. eCollection 2020 Aug. PLoS Negl Trop Dis. 2020. PMID: 32866199 Free PMC article.
-
Insulin-Like Peptide Signaling in Mosquitoes: The Road Behind and the Road Ahead.Front Endocrinol (Lausanne). 2019 Mar 22;10:166. doi: 10.3389/fendo.2019.00166. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30984106 Free PMC article. Review.
References
-
- Antonova Y., Arik A. J., Moore W., Riehle M. R., Brown M. R. (2012). Insulin-like peptides: structure, signaling, and function. In Insect Endocrinology (ed. Gilbert L. I.), pp. 63-92 Boston, MA: Academic Press;
-
- Balendran A., Currie R., Armstrong C. G., Avruch J., Alessi D. R. (1999). Evidence that 3-phosphoinositide-dependent protein kinase-1 mediates phosphorylation of p70 S6 kinase in vivo at Thr-412 as well as Thr-252. J. Biol. Chem. 274, 37400-37406 - PubMed
-
- Corby-Harris V., Drexler A., Watkins de Jong L., Antonova Y., Pakpour N., Ziegler R., Ramberg F., Lewis E. E., Brown J. M., Luckhart S., et al. (2010). Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes. PLoS Pathog. 6, e1001003 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

