Binding to the DNA minor groove by heterocyclic dications: from AT-specific monomers to GC recognition with dimers
- PMID: 23255206
- PMCID: PMC3539175
- DOI: 10.1002/0471142700.nc0808s51
Binding to the DNA minor groove by heterocyclic dications: from AT-specific monomers to GC recognition with dimers
Abstract
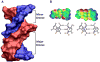
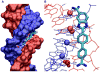
Compounds that bind in the DNA minor groove have provided critical information on DNA molecular recognition, have found extensive uses in biotechnology, and are providing clinically useful drugs against diseases as diverse as cancer and sleeping sickness. This review focuses on the development of clinically useful heterocyclic diamidine minor groove binders. These compounds have shown us that the classical model for minor groove binding in AT DNA sequences must be expanded in several ways: compounds with nonstandard shapes can bind strongly to the groove, water can be directly incorporated into the minor groove complex in an interfacial interaction, and the compounds can form cooperative stacked dimers to recognize GC and mixed AT/GC base pair sequences.
© 2012 by John Wiley & Sons, Inc.
Figures








References
-
- Bailly C, Tardy C, Wang L, Armitage B, Hopkins K, Kumar A, Schuster GB, Boykin DW, Wilson WD. Recognition of ATGA sequences by the unfused aromatic dication DB293 forming stacked dimers in the DNA minor groove. Biochemistry. 2001;40:9770–9779. - PubMed
-
- Bajic M, Kumar A, Boykin DW. Synthesis of 2,5-bis-(4-cyanophenyl)-furan. Heterocycl Commun. 1996;2:135–140.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

