Children's Oncology Group's 2013 blueprint for research: acute myeloid leukemia
- PMID: 23255301
- PMCID: PMC4605815
- DOI: 10.1002/pbc.24432
Children's Oncology Group's 2013 blueprint for research: acute myeloid leukemia
Abstract
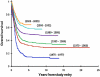
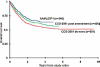
For the 365 children diagnosed with acute myeloid leukemia in the US annually, 5-year survival for patients on COG trials with low, intermediate, and high risk disease is 83%, 62%, and 23%, respectively. Recent advances include improved therapeutic stratification, improved survival with dose intensification, and further elucidation of the heterogeneity specific to childhood AML. These discoveries now guide current strategy incorporating targeted agents to pathways specific to childhood AML as well as evaluating methods to increase the sensitivity of the leukemic stem cell, first in Phase II feasibility trials followed by Phase III efficacy trials of the most promising agents. Acute myeloid leukemia in children, though with similar subgroups to adults, remains uniquely different based upon quite different prevalence of subtypes as well as overall response to therapy. The Children's Oncology Group's research agenda builds upon earlier efforts to better elucidate the leukemogenic steps distinct to childhood AML in order to more scientifically develop and test novel therapeutic approaches to the treatment and ultimate cure for children with this disorder. Pediatr Blood Cancer 2013; 60: 964-971. © 2012 Wiley Periodicals, Inc.
Copyright © 2012 Wiley Periodicals, Inc.
Figures
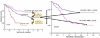
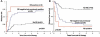
References
-
- Creutzig U, van den Heuvel-Eibrink M, Gibson B, et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: Recommendations from an international expert panel. Blood. 2012;120:3187–3205. - PubMed
-
- Cooper TM, Franklin J, Gerbing R, et al. AAML03P1, a pilot study of the safety of Gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia. Cancer. 2012;118:761–769. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

