Spliceosome integrity is defective in the motor neuron diseases ALS and SMA
- PMID: 23255347
- PMCID: PMC3569639
- DOI: 10.1002/emmm.201202303
Spliceosome integrity is defective in the motor neuron diseases ALS and SMA
Abstract
Two motor neuron diseases, amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA), are caused by distinct genes involved in RNA metabolism, TDP-43 and FUS/TLS, and SMN, respectively. However, whether there is a shared defective mechanism in RNA metabolism common to these two diseases remains unclear. Here, we show that TDP-43 and FUS/TLS localize in nuclear Gems through an association with SMN, and that all three proteins function in spliceosome maintenance. We also show that in ALS, Gems are lost, U snRNA levels are up-regulated and spliceosomal U snRNPs abnormally and extensively accumulate in motor neuron nuclei, but not in the temporal lobe of FTLD with TDP-43 pathology. This aberrant accumulation of U snRNAs in ALS motor neurons is in direct contrast to SMA motor neurons, which show reduced amounts of U snRNAs, while both have defects in the spliceosome. These findings indicate that a profound loss of spliceosome integrity is a critical mechanism common to neurodegeneration in ALS and SMA, and may explain cell-type specific vulnerability of motor neurons.
Copyright © 2013 The Authors. Published by John Wiley and Sons, Ltd on behalf of EMBO.
Figures

A–C. Hela cells were immunostained with antibodies against TDP-43 and nuclear domain markers. Magnified images showing colocalization of TDP-43 and nuclear markers (upper right). (A,B) Costaining of TDP-43 and components of Gems. TDP-43 was extensively concentrated in Gems marked by SMN (A, arrows) or Gemin8 (B, arrows). (C) Costaining of Coilin, a Cajal body marker, and TDP-43. TDP-43 was concentrated in Cajal bodies marked by Coilin (arrows). Bars: 10 µm.
D,E. TDP-43 localized in Gems of neuronal cell line SH-SY5Y (D, arrows) and primary cultured mouse hippocampal neurons (E, arrows). Bars: 10 µm.
F. Costaining of FUS/TLS and Gemin8 in primary cultured mouse hippocampal neurons. FUS/TLS localized in Gem (arrows). Bars: 10 µm.
G. Hela cells were treated with siRNAs for TDP-43 or control to deplete TDP-43, and immunostained for SMN and TDP-43. Gems are lost in cells with no TDP-43 expression (arrows), whereas they remain in cells with low TDP-43 expression (arrowheads). Bars: 10 µm.
H. Quantification of Gem numbers in Hela cells treated with siRNAs shown in G. Cells with no TDP-43 expression in immunostaining were shown as knockdown (KD), whereas cells with low TDP-43 expression level in immunostaining were shown as mild KD. Means for number of Gems are 2.857 (Control, n = 21), 2.947 (mild KD, n = 19) and 0.1 (KD, n = 20) (Control vs KD: p < 0.0001).
I. DIV 21 hippocampal neurons form FUS−/− mice or littermates were stained for SMN to analyze the requirement of FUS/TLS for Gem formation. Bars: 10 µm.
J. Quantification of Gems positive for SMN. Means for number of Gems are 0.4026 (FUS+/+, n = 77) and 0.02667 (FUS−/−, n = 75) (p < 0.0001).

A. A schematic diagram of C-terminal 3XFLAG-tagged expression constructs for TDP-43 used in this study.
B. The latter half of the C-terminal glycine-rich region of TDP-43 was important for the proper localization to Gems. Hela cells were transfected with TDP-43-3XFLAG or indicated mutants, and stained with anti-SMN and anti-FLAG antibodies. Co-localization of TDP-43 and SMN was assessed by confocal microscope, numbers of TDP-43-positive Gems and -negative Gems were counted. More than 100 Gems were counted for each construct, and the localization to Gem (%) was defined as TDP-43-postive Gems per total Gems (%). To eliminate variation in the number of Gems per nucleus, cloned Hela cells were used. The average and error bars from three independent experiments were plotted.
C,D. The SMN/Gemin8/FUS interactions with TDP-43 were dependent on the TDP-43 C-terminus. TDP-43-3xFLAG mutants were expressed in Hela cells, and the TDP-43 interacting proteins were immunoprecipitated using an anti-FLAG antibody and identified by Western blot analysis using the specific antibodies as indicated. Asterisks indicate degraded FLAG-tagged TDP-43 proteins.
E. A schematic diagram of N-terminal 3XFLAG-tagged expression constructs for FUS/TLS used in this study.
F. 3xFLAG-FUS/TLS mutants were expressed in Hela cells, and the FUS/TLS interacting proteins were immunoprecipitated using an anti-FLAG antibody and identified by Western blot analysis using the specific antibodies as indicated.
G. Mutations in Tudor domain of SMN1 decreased association of TDP-43 and FUS/TLS. HA-tagged human SMN1 and its mutants were expressed in Hela cells, and SMN interacting proteins were immunoprecipitated using an anti-HA antibody and identified by Western blot analysis using the specific antibodies as indicated.
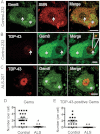
Immunostaining of Gems in human spinal cord motor neurons. Paraffin-fixed post-mortem spinal cords from patients with neurological diseases other than ALS were analysed under a confocal microscope for the presence of Gems with antibodies against SMN and Gemin8 (Gem8) (arrows). Note that autofluorescence derived from lipofuscin was observed in the cytoplasm (asterisks). Bars: 10 µm.
Coimmunostaining of TDP-43 and SMN indicating the presence of TDP-43-positive Gems in human spinal cord motor neurons (arrows). Bars: 10 µm.
Coimmunostaining of TDP-43 and Gemin8 in remaining motor neurons of ALS spinal cords. TDP-43 is localized in the nucleus. Bars: 10 µm.
Nuclear foci with a significant concentration with Gemin8 and SMN were defined as Gem, and numbers of Gems in motor neurons from three control patients (N = 21) or three ALS patients (N = 25) were counted. Means are 2.476 and 0.08, respectively (p < 0.0001).
Nuclear foci with a significant concentration with Gemin8 as determined in (D) were defined as Gem, and numbers of TDP-43-positive Gems in motor neurons of spinal cords with control disease (N = 19) or ALS (N = 18). Means are 1.895 and 0.056, respectively (p < 0.0001).
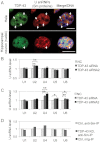
A. TDP-43 colocalized with U snRNPs that were marked with an anti-dimethylated Sm proteins antibody (Y12) in the nuclei of Hela cells and primary cultured mouse hippocampal neurons. Note that U snRNPs and TDP-43 were concentrated in the same nuclear bodies (arrows). Bar: 10 µm.
B,C. U snRNA levels in Hela cells (B) or SH-SY5Y cells (C) treated with siRNAs for TDP-43 or control were determined by quantitative RT-PCR. Average from three independent experiments with transfections performed in triplicate were plotted (Bars: standard errors, *p < 0.05, **p < 0.01, Student's t-test).
D. Mature U snRNP-associated U snRNA levels in Hela cells treated with siRNAs for TDP-43 or control. U snRNA levels were determined by quantitative RT-PCR from the RNAs isolated from mature U snRNP complex which was immunopurified using anti-Sm proteins antibody (Y12) as described in Materials and Methods.
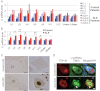
The RNAs were isolated from cervical spinal cords of four ALS patients (ALS-1 to ALS-4) or five control patients with other neurological disease (C-1 to C-5), and U snRNA levels were determined by quantitative RT-PCR as in Fig 4. Detailed clinical information is listed in Supporting Information Table S1.
Mean U snRNA levels of control and ALS patients determined in A were plotted. Average amounts of U snRNAs from the five control patients were used for normalization. Robust U snRNA misregulation was observed in ALS patients. (Bars: standard errors, *p < 0.05, **p < 0.01, Student's t-test).
Immunohistochemistry of spinal cords from patients with control disease or ALS using an anti-2,2,7-trimethylguanosine (TMG) antibody, which recognized the 5′ cap structure of snRNA. Boxed areas were shown as the magnified images (Right panels). Bars: 500 µm (left), 20 µm (right).
Immunofluorescence staining of spinal motor neurons using anti-TMG and anti-TDP-43 antibodies. Note that strong accumulation of U snRNAs in nucleus of motor neurons from ALS patients (arrowheads). Asterisks show autofluorescence derived from lipofuscin in the cytoplasm. Bars: 10 µm.
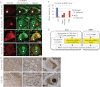
A–D. Immunofluorescent staining of TDP-43 and U snRNPs using an anti-Sm proteins antibody (Y12) in spinal cord motor neurons from patients with control diseases (A) or ALS (B–D). Arrow shows colocalization of the Y12 antigen and TDP-43 (A). The Y12 antigen accumulated in nuclei of ALS motor neurons with TDP-43 mislocalization (B–D). Arrowheads (B–D) show accumulated U snRNPs in motor neuron nuclei from ALS patients. Asterisks show autofluorescence derived from lipofuscin in the cytoplasm. Bars: 10 µm.
E. Immunohistochemistry of U snRNPs using an anti-Sm proteins antibody (Y12) in spinal cord motor neurons from patients with control diseases or ALS. Boxed areas in the left and middle panels were shown as the magnified images in the middle and right panels, respectively. Bars: 500 µm (left), 100 µm (middle), 20 µm (right).
F. Quantification of U snRNP immunofluorescence levels in motor neuron nuclei. Motor neurons from four control patients (blue, N = 35) and three ALS patients (red, N = 105) were analysed for U snRNP fluorescence intensity in their nuclei. The level of fluorescence intensity of U snRNPs in nuclei compared with cytoplasm were classified as low (nuclear U snRNP level is lower than cytosol), intermediate (nuclear U snRNP level is equal to cytosol), or high (nuclear U snRNP level is higher than cytosol), and plotted. Representative images of motor neurons showing low, intermediate or high nuclear U snRNP levels are shown in Supporting Information Fig S7.
G. Model for mechanism underlying neurodegeneration in ALS with dysfunction of TDP-43 in comparison to SMA. In this study, we show that TDP-43 localizes in nuclear Gem through association with SMN complex, and is involved in maintenance of spliceosome through controlling the levels of U snRNAs. In ALS patients and SMA mice, U snRNA levels are misregulated in spinal cord. Intriguingly, accumulation of U snRNA is seen in ALS, while loss of U snRNAs is reported in SMA. Moreover, Gems are lost and spliceosomal U snRNPs abnormally accumulate in nuclei of motor neurons from ALS patients. These findings indicate collapse of spliceosome integrity is the critical process common to motor neuron degeneration in ALS and SMA, and may explain cell-type specific vulnerability in motor neurons.
References
-
- Andersen PM, Al-Chalabi A. Clinical genetics of amyotrophic lateral sclerosis: what do we really know. Nat Rev Neurol. 2011;7:603–615. - PubMed
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

