Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern
- PMID: 23255361
- PMCID: PMC3618989
- DOI: 10.4049/jimmunol.1202805
Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern
Abstract
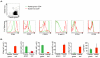
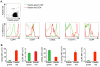
Several populations of memory T cells have been described that differ in their migration and function. In this study, we have identified a unique subset of memory T cells, which we have named recirculating memory T cells (T(RCM)). By exposing Kaede transgenic mouse skin to violet light, we tracked the fate of cutaneous T cells. One population of memory CD4(+) T cells remained in the skin. A second population migrated from the skin into draining lymph nodes (LNs) in a CCR7-dependent manner. These migrating CD4(+) T cells expressed a novel cell surface phenotype (CCR7(int/+)CD62L(int)CD69(-)CD103(+/-) E-selectin ligands(+)) that is distinct from memory T cell subsets described to date. Unlike memory T cell subsets that remain resident within tissues long-term, or that migrate either exclusively between lymphoid tissues or into peripheral nonlymphoid sites, CD4(+) T(RCM) migrate from the skin into draining LNs. From the draining LNs, CD4(+) T(RCM) reenter into the circulation, distal LNs, and sites of non-specific cutaneous inflammation. In addition, CD4(+) T(RCM) upregulated CD40L and secreted IL-2 following polyclonal stimulation. Our results identify a novel subset of recirculating memory CD4(+) T cells equipped to deliver help to both distal lymphoid and cutaneous tissues.
Figures



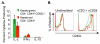
References
-
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. - PubMed
-
- Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. - PubMed
-
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. - PubMed
-
- Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

