Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms
- PMID: 23255504
- PMCID: PMC3630464
- DOI: 10.1002/humu.22261
Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms
Abstract
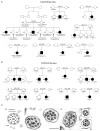
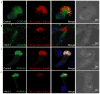
Primary ciliary dyskinesia (PCD) is a genetically heterogeneous disorder caused by cilia and sperm dysmotility. About 12% of cases show perturbed 9+2 microtubule cilia structure and inner dynein arm (IDA) loss, historically termed "radial spoke defect." We sequenced CCDC39 and CCDC40 in 54 "radial spoke defect" families, as these are the two genes identified so far to cause this defect. We discovered biallelic mutations in a remarkable 69% (37/54) of families, including identification of 25 (19 novel) mutant alleles (12 in CCDC39 and 13 in CCDC40). All the mutations were nonsense, splice, and frameshift predicting early protein truncation, which suggests this defect is caused by "null" alleles conferring complete protein loss. Most families (73%; 27/37) had homozygous mutations, including families from outbred populations. A major putative hotspot mutation was identified, CCDC40 c.248delC, as well as several other possible hotspot mutations. Together, these findings highlight the key role of CCDC39 and CCDC40 in PCD with axonemal disorganization and IDA loss, and these genes represent major candidates for genetic testing in families affected by this ciliary phenotype. We show that radial spoke structures are largely intact in these patients and propose this ciliary ultrastructural abnormality be referred to as "IDA and microtubular disorganisation defect," rather than "radial spoke defect."
© 2012 Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- Antonelli M, Modesti A, De AM, Marcolini P, Lucarelli N, Crifo S. Immotile cilia syndrome: radial spokes deficiency in a patient with Kartagener’s triad. Acta Paediatr Scand. 1981;70:571–573. - PubMed
-
- Bartoloni L, Blouin JL, Pan Y, Gehrig C, Maiti AK, Scamuffa N, Rossier C, Jorissen M, Armengot M, Meeks M, Mitchison HM, Chung EM, DeLozier-Blanchet CD, Craigen WJ, Antonarakis SE. Mutations in the DNAH11 (axonemal heavy chain dynein type 11) gene cause one form of situs inversus totalis and most likely primary ciliary dyskinesia. Proc Natl Acad Sci U S A. 2002;99:10282–10286. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ULITR000083/PHS HHS/United States
- UL1 TR000154/TR/NCATS NIH HHS/United States
- MR/K000608/1/MRC_/Medical Research Council/United Kingdom
- NF-SI-0510-10268/DH_/Department of Health/United Kingdom
- 5 R01HL071798/HL/NHLBI NIH HHS/United States
- U54 HL096458/HL/NHLBI NIH HHS/United States
- WT091310/WT_/Wellcome Trust/United Kingdom
- T32 HL007106/HL/NHLBI NIH HHS/United States
- R01 HL071798/HL/NHLBI NIH HHS/United States
- 090532/WT_/Wellcome Trust/United Kingdom
- RG/10/13/28570/BHF_/British Heart Foundation/United Kingdom
- 091551/WT_/Wellcome Trust/United Kingdom
- M01 RR000046/RR/NCRR NIH HHS/United States
- 5 U54 HL096458-06/HL/NHLBI NIH HHS/United States
- UL1 TR000083/TR/NCATS NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

