Adiponectin receptors form homomers and heteromers exhibiting distinct ligand binding and intracellular signaling properties
- PMID: 23255609
- PMCID: PMC3561534
- DOI: 10.1074/jbc.M112.404624
Adiponectin receptors form homomers and heteromers exhibiting distinct ligand binding and intracellular signaling properties
Abstract
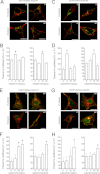
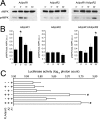
Adiponectin binds to two widely expressed receptors (AdipoR1 and AdipoR2) that contain seven transmembrane domains but, unlike G-protein coupled receptors, present an extracellular C terminus and a cytosolic N terminus. Recently, AdipoR1 was found to associate in high order complexes. However, it is still unknown whether AdipoR2 may also form homomers or heteromers with AdipoR1 or if such interactions may be functionally relevant. Herein, we have analyzed the oligomerization pattern of AdipoRs by FRET and immunoprecipitation and evaluated both the internalization of AdipoRs in response to various adiponectin isoforms and the effect of adiponectin binding to different AdipoR combinations on AMP-activated protein kinase phosphorylation and peroxisome proliferator-activated receptor α activation. Transfection of HEK293AD cells with AdipoR1 and AdipoR2 showed that both receptors colocalize at both the plasma membrane and the endoplasmic reticulum. Co-transfection with the different AdipoR pairs yielded high FRET efficiencies in non-stimulated cells, which indicates that AdipoR1 and AdipoR2 form homo- and heteromeric complexes under resting conditions. Live FRET imaging suggested that both homo- and heteromeric AdipoR complexes dissociate in response to adiponectin, but heteromers separate faster than homomers. Finally, phosphorylation of AMP-activated protein kinase in response to adiponectin was delayed in cells wherein heteromer formation was favored. In sum, our findings indicate that AdipoR1 and AdipoR2 form homo- and heteromers that present unique interaction behaviors and signaling properties. This raises the possibility that the pleiotropic, tissue-dependent functions of adiponectin depend on the expression levels of AdipoR1 and AdipoR2 and, therefore, on the steady-state proportion of homo- and heteromeric complexes.
Figures





References
-
- Rajala M. W., Scherer P. E. (2003) Minireview. The adipocyte. At the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology 144, 3765–3773 - PubMed
-
- Scherer P. E., Williams S., Fogliano M., Baldini G., Lodish H. F. (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 - PubMed
-
- Hu E., Liang P., Spiegelman B. M. (1996) AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 271, 10697–10703 - PubMed
-
- Maeda K., Okubo K., Shimomura I., Funahashi T., Matsuzawa Y., Matsubara K. (1996) cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose most abundant gene transcript 1). Biochem. Biophys. Res. Commun. 221, 286–289 - PubMed
-
- Nakano Y., Tobe T., Choi-Miura N. H., Mazda T., Tomita M. (1996) Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J. Biochem. 120, 803–812 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

