Reversed synaptic effects of hypocretin and NPY mediated by excitatory GABA-dependent synaptic activity in developing MCH neurons
- PMID: 23255725
- PMCID: PMC3602944
- DOI: 10.1152/jn.00522.2012
Reversed synaptic effects of hypocretin and NPY mediated by excitatory GABA-dependent synaptic activity in developing MCH neurons
Abstract
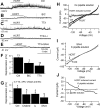
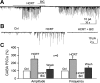
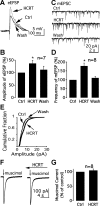
In mature neurons, GABA is the primary inhibitory neurotransmitter. In contrast, in developing neurons, GABA exerts excitatory actions, and in some neurons GABA-mediated excitatory synaptic activity is more prevalent than glutamate-mediated excitation. Hypothalamic neuropeptides that modulate cognitive arousal and energy homeostasis, hypocretin/orexin and neuropeptide Y (NPY), evoked reversed effects on synaptic actions that were dependent on presynaptic GABA release onto melanin-concentrating hormone (MCH) neurons. MCH neurons were identified by selective green fluorescent protein (GFP) expression in transgenic mice. In adults, hypocretin increased GABA release leading to reduced excitation. In contrast, in the developing brain as studied here with analysis of miniature excitatory postsynaptic currents, paired-pulse ratios, and evoked potentials, hypocretin acted presynaptically to enhance the excitatory actions of GABA. The ability of hypocretin to enhance GABA release increases inhibition in adult neurons but paradoxically enhances excitation in developing MCH neurons. In contrast, NPY attenuation of GABA release reduced inhibition in mature neurons but enhanced inhibition during development by attenuating GABA excitation. Both hypocretin and NPY also evoked direct actions on developing MCH neurons. Hypocretin excited MCH cells by activating a sodium-calcium exchanger and by reducing potassium currents; NPY reduced activity by increasing an inwardly rectifying potassium current. These data for the first time show that both hypocretin and NPY receptors are functional presynaptically during early postnatal hypothalamic development and that both neuropeptides modulate GABA actions during development with a valence of enhanced excitation or inhibition opposite to that of the adult state, potentially allowing neuropeptide modulation of use-dependent synapse stabilization.
Figures



Similar articles
-
Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus.J Physiol. 2001 May 15;533(Pt 1):237-52. doi: 10.1111/j.1469-7793.2001.0237b.x. J Physiol. 2001. PMID: 11351031 Free PMC article.
-
Interactions of neuropeptide Y, hypocretin-I (orexin A) and melanin-concentrating hormone on feeding in rats.Brain Res. 2002 Jul 19;944(1-2):232-8. doi: 10.1016/s0006-8993(02)02941-4. Brain Res. 2002. PMID: 12106685
-
Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus.J Neurosci. 2008 Sep 10;28(37):9101-10. doi: 10.1523/JNEUROSCI.1766-08.2008. J Neurosci. 2008. PMID: 18784290 Free PMC article.
-
MCH and feeding behavior-interaction with peptidic network.Peptides. 2009 Nov;30(11):2045-51. doi: 10.1016/j.peptides.2009.07.008. Epub 2009 Jul 18. Peptides. 2009. PMID: 19619600 Review.
-
Electrophysiological effects of MCH on neurons in the hypothalamus.Peptides. 2009 Nov;30(11):2025-30. doi: 10.1016/j.peptides.2009.05.006. Epub 2009 May 20. Peptides. 2009. PMID: 19463877 Free PMC article. Review.
Cited by
-
Heightened stress response and cognitive impairment after repeated neonatal sevoflurane exposures might be linked to excessive GABAAR-mediated depolarization.J Anesth. 2016 Oct;30(5):834-41. doi: 10.1007/s00540-016-2215-0. Epub 2016 Jul 19. J Anesth. 2016. PMID: 27435414
-
Early expression of hypocretin/orexin in the chick embryo brain.PLoS One. 2014 Sep 4;9(9):e106977. doi: 10.1371/journal.pone.0106977. eCollection 2014. PLoS One. 2014. PMID: 25188307 Free PMC article.
-
Orexin/Hypocretin Signaling.Curr Top Behav Neurosci. 2017;33:17-50. doi: 10.1007/7854_2016_49. Curr Top Behav Neurosci. 2017. PMID: 27909990 Review.
-
Anesthesia with sevoflurane in neonatal rats: Developmental neuroendocrine abnormalities and alleviating effects of the corticosteroid and Cl(-) importer antagonists.Psychoneuroendocrinology. 2015 Oct;60:173-81. doi: 10.1016/j.psyneuen.2015.06.016. Epub 2015 Jun 25. Psychoneuroendocrinology. 2015. PMID: 26150359 Free PMC article.
References
-
- Beloosesky R, Gayle DA, Amidi F, Ahanya SN, Desai M, Ross MG. Ontogenic expression of putative feeding peptides in the rat fetal brain and placenta. Nutr Neurosci 9: 33–40, 2006 - PubMed
-
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol 319: 218–245, 1992 - PubMed
-
- Botchkina GI, Morin LP. Organization of permanent and transient neuropeptide Y-immunoreactive neuron groups and fiber systems in the developing hamster diencephalon. J Comp Neurol 357: 573–602, 1995 - PubMed
-
- Broberger C, De Lecea L, Sutcliffe JG, Hökfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol 402: 460–474, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

