Pretreatment diffusion-weighted and dynamic contrast-enhanced MRI for prediction of local treatment response in squamous cell carcinomas of the head and neck
- PMID: 23255739
- PMCID: PMC3549615
- DOI: 10.2214/AJR.12.9432
Pretreatment diffusion-weighted and dynamic contrast-enhanced MRI for prediction of local treatment response in squamous cell carcinomas of the head and neck
Abstract
Objective: The objective of our study was to predict response to chemoradiation therapy in patients with head and neck squamous cell carcinoma (HNSCC) by combined use of diffusion-weighted imaging (DWI) and high-spatial-resolution, high-temporal-resolution dynamic contrast-enhanced MRI (DCE-MRI) parameters from primary tumors and metastatic nodes.
Subjects and methods: Thirty-two patients underwent pretreatment DWI and DCE-MRI using a modified radial imaging sequence. Postprocessing of data included motion-correction algorithms to reduce motion artifacts. The median apparent diffusion coefficient (ADC), volume transfer constant (K(trans)), extracellular extravascular volume fraction (v(e)), and plasma volume fraction (v(p)) were computed from primary tumors and nodal masses. The quality of the DCE-MRI maps was estimated using a threshold median chi-square value of 0.10 or less. Multivariate logistic regression and receiver operating characteristic curve analyses were used to determine the best model to discriminate responders from nonresponders.
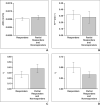
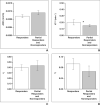
Results: Acceptable χ(2) values were observed from 84% of primary tumors and 100% of nodal masses. Five patients with unsatisfactory DCE-MRI data were excluded and DCE-MRI data for three patients who died of unrelated causes were censored from analysis. The median follow-up for the remaining patients (n = 24) was 23.72 months. When ADC and DCE-MRI parameters (K(trans), v(e), v(p)) from both primary tumors and nodal masses were incorporated into multivariate logistic regression analyses, a considerably higher discriminative accuracy (area under the curve [AUC] = 0.85) with a sensitivity of 81.3% and specificity of 75% was observed in differentiating responders (n = 16) from nonresponders (n = 8).
Conclusion: The combined use of DWI and DCE-MRI parameters from both primary tumors and nodal masses may aid in prediction of response to chemoradiation therapy in patients with HNSCC.
Figures



References
-
- Lee J, Moon C. Current status of experimental therapeutics for head and neck cancer. Exp Biol Med (Maywood) 2011;236:375–389. - PubMed
-
- McCollum AD, Burrell SC, Haddad RI, et al. Positron emission tomography with 18F-fluorode-oxyglucose to predict pathologic response after induction chemotherapy and definitive chemoradiotherapy in head and neck cancer. Head Neck. 2004;26:890–896. - PubMed
-
- Lyford-Pike S, Ha PK, Jacene HA, Saunders JR, Tufano RP. Limitations of PET/CT in determining need for neck dissection after primary chemoradiation for advanced head and neck squamous cell carcinoma. ORL J Otorhinolaryngol Relat Spec. 2009;71:251–256. - PubMed
-
- Machiels JP, Henry S, Zanetta S, et al. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006-01. J Clin Oncol. 2010;28:21–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

