Tropism and infectivity of influenza virus, including highly pathogenic avian H5N1 virus, in ferret tracheal differentiated primary epithelial cell cultures
- PMID: 23255802
- PMCID: PMC3571379
- DOI: 10.1128/JVI.02885-12
Tropism and infectivity of influenza virus, including highly pathogenic avian H5N1 virus, in ferret tracheal differentiated primary epithelial cell cultures
Abstract
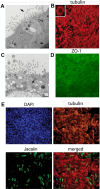
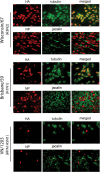
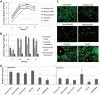
Tropism and adaptation of influenza viruses to new hosts is partly dependent on the distribution of the sialic acid (SA) receptors to which the viral hemagglutinin (HA) binds. Ferrets have been established as a valuable in vivo model of influenza virus pathogenesis and transmission because of similarities to humans in the distribution of HA receptors and in clinical signs of infection. In this study, we developed a ferret tracheal differentiated primary epithelial cell culture model that consisted of a layered epithelium structure with ciliated and nonciliated cells on its apical surface. We found that human-like (α2,6-linked) receptors predominated on ciliated cells, whereas avian-like (α2,3-linked) receptors, which were less abundant, were presented on nonciliated cells. When we compared the tropism and infectivity of three human (H1 and H3) and two avian (H1 and H5) influenza viruses, we observed that the human influenza viruses primarily infected ciliated cells and replicated efficiently, whereas a highly pathogenic avian H5N1 virus (A/Vietnam/1203/2004) replicated efficiently within nonciliated cells despite a low initial infection rate. Furthermore, compared to other influenza viruses tested, VN/1203 virus replicated more efficiently in cells isolated from the lower trachea and at a higher temperature (37°C) compared to a lower temperature (33°C). VN/1203 virus infection also induced higher levels of immune mediator genes and cell death, and virus was recovered from the basolateral side of the cell monolayer. This ferret tracheal differentiated primary epithelial cell culture system provides a valuable in vitro model for studying cellular tropism, infectivity, and the pathogenesis of influenza viruses.
Figures

References
-
- Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. 2000. Clinical signs and symptoms predicting influenza infection. Arch. Intern. Med. 160:3243–3247 - PubMed
-
- Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. 2006. Avian flu: influenza virus receptors in the human airway. Nature 440:435–436 - PubMed
-
- Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, Hayden FG, Nguyen DH, de Jong MD, Naghdaliyev A, Peiris JS, Shindo N, Soeroso S, Uyeki TM. 2008. Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 358:261–273 - PubMed
-
- de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, do Q Ha, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12:1203–1207 - PMC - PubMed
-
- Baum LG, Paulson JC. 1990. Sialyloligosaccharides of the respiratory epithelium in the selection of human influenza virus receptor specificity. Acta Histochem. Suppl. 40:35–38 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

