Tra2-mediated recognition of HIV-1 5' splice site D3 as a key factor in the processing of vpr mRNA
- PMID: 23255807
- PMCID: PMC3571381
- DOI: 10.1128/JVI.02756-12
Tra2-mediated recognition of HIV-1 5' splice site D3 as a key factor in the processing of vpr mRNA
Abstract
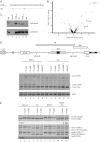
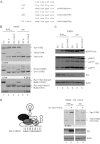
Small noncoding HIV-1 leader exon 3 is defined by its splice sites A2 and D3. While 3' splice site (3'ss) A2 needs to be activated for vpr mRNA formation, the location of the vpr start codon within downstream intron 3 requires silencing of splicing at 5'ss D3. Here we show that the inclusion of both HIV-1 exon 3 and vpr mRNA processing is promoted by an exonic splicing enhancer (ESE(vpr)) localized between exonic splicing silencer ESSV and 5'ss D3. The ESE(vpr) sequence was found to be bound by members of the Transformer 2 (Tra2) protein family. Coexpression of these proteins in provirus-transfected cells led to an increase in the levels of exon 3 inclusion, confirming that they act through ESE(vpr). Further analyses revealed that ESE(vpr) supports the binding of U1 snRNA at 5'ss D3, allowing bridging interactions across the upstream exon with 3'ss A2. In line with this, an increase or decrease in the complementarity of 5'ss D3 to the 5' end of U1 snRNA was accompanied by a higher or lower vpr expression level. Activation of 3'ss A2 through the proposed bridging interactions, however, was not dependent on the splicing competence of 5'ss D3 because rendering it splicing defective but still competent for efficient U1 snRNA binding maintained the enhancing function of D3. Therefore, we propose that splicing at 3'ss A2 occurs temporally between the binding of U1 snRNA and splicing at D3.
Figures





Similar articles
-
A suboptimal 5' splice site downstream of HIV-1 splice site A1 is required for unspliced viral mRNA accumulation and efficient virus replication.Retrovirology. 2006 Feb 3;3:10. doi: 10.1186/1742-4690-3-10. Retrovirology. 2006. PMID: 16457729 Free PMC article.
-
Negative and positive mRNA splicing elements act competitively to regulate human immunodeficiency virus type 1 vif gene expression.J Virol. 2008 Apr;82(8):3921-31. doi: 10.1128/JVI.01558-07. Epub 2008 Feb 13. J Virol. 2008. PMID: 18272582 Free PMC article.
-
Regulation of Vif mRNA splicing by human immunodeficiency virus type 1 requires 5' splice site D2 and an exonic splicing enhancer to counteract cellular restriction factor APOBEC3G.J Virol. 2009 Jun;83(12):6067-78. doi: 10.1128/JVI.02231-08. Epub 2009 Apr 8. J Virol. 2009. PMID: 19357165 Free PMC article.
-
A novel role of U1 snRNP: Splice site selection from a distance.Biochim Biophys Acta Gene Regul Mech. 2019 Jun;1862(6):634-642. doi: 10.1016/j.bbagrm.2019.04.004. Epub 2019 Apr 28. Biochim Biophys Acta Gene Regul Mech. 2019. PMID: 31042550 Free PMC article. Review.
-
Pick one, but be quick: 5' splice sites and the problems of too many choices.Genes Dev. 2013 Jan 15;27(2):129-44. doi: 10.1101/gad.209759.112. Genes Dev. 2013. PMID: 23348838 Free PMC article. Review.
Cited by
-
Post-Transcriptional HIV-1 Latency: A Promising Target for Therapy?Viruses. 2024 Apr 24;16(5):666. doi: 10.3390/v16050666. Viruses. 2024. PMID: 38793548 Free PMC article. Review.
-
Succession of splicing regulatory elements determines cryptic 5΄ss functionality.Nucleic Acids Res. 2017 Apr 20;45(7):4202-4216. doi: 10.1093/nar/gkw1317. Nucleic Acids Res. 2017. PMID: 28039323 Free PMC article.
-
Host RNA-Binding Proteins as Regulators of HIV-1 Replication.Viruses. 2024 Dec 31;17(1):43. doi: 10.3390/v17010043. Viruses. 2024. PMID: 39861832 Free PMC article. Review.
-
Balanced splicing at the Tat-specific HIV-1 3'ss A3 is critical for HIV-1 replication.Retrovirology. 2015 Mar 28;12:29. doi: 10.1186/s12977-015-0154-8. Retrovirology. 2015. PMID: 25889056 Free PMC article.
-
SRSF1 acts as an IFN-I-regulated cellular dependency factor decisively affecting HIV-1 post-integration steps.Front Immunol. 2022 Nov 15;13:935800. doi: 10.3389/fimmu.2022.935800. eCollection 2022. Front Immunol. 2022. PMID: 36458014 Free PMC article.
References
-
- Frankel AD, Young JA. 1998. HIV-1: fifteen proteins and an RNA. Annu. Rev. Biochem. 67:1–25 - PubMed
-
- Krummheuer J, Johnson AT, Hauber I, Kammler S, Anderson JL, Hauber J, Purcell DF, Schaal H. 2007. A minimal uORF within the HIV-1 vpu leader allows efficient translation initiation at the downstream env AUG. Virology 363:261–271 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

