How vision and movement combine in the hippocampal place code
- PMID: 23256159
- PMCID: PMC3538268
- DOI: 10.1073/pnas.1215834110
How vision and movement combine in the hippocampal place code
Abstract
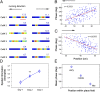
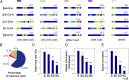
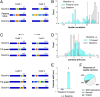
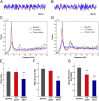
How do external environmental and internal movement-related information combine to tell us where we are? We examined the neural representation of environmental location provided by hippocampal place cells while mice navigated a virtual reality environment in which both types of information could be manipulated. Extracellular recordings were made from region CA1 of head-fixed mice navigating a virtual linear track and running in a similar real environment. Despite the absence of vestibular motion signals, normal place cell firing and theta rhythmicity were found. Visual information alone was sufficient for localized firing in 25% of place cells and to maintain a local field potential theta rhythm (but with significantly reduced power). Additional movement-related information was required for normally localized firing by the remaining 75% of place cells. Trials in which movement and visual information were put into conflict showed that they combined nonlinearly to control firing location, and that the relative influence of movement versus visual information varied widely across place cells. However, within this heterogeneity, the behavior of fully half of the place cells conformed to a model of path integration in which the presence of visual cues at the start of each run together with subsequent movement-related updating of position was sufficient to maintain normal fields.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–175. - PubMed
-
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Oxford Univ Press; 1978.
-
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261(5124):1055–1058. - PubMed
-
- Mittelstaedt H, Mittelstaedt ML. Mechanismen der orientierung ohne richtende aussenreize. Fortschr Zool. 1973;21:46–58.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

