Salvage chemoradiotherapy after primary chemotherapy for locally advanced pancreatic cancer: a single-institution retrospective analysis
- PMID: 23256481
- PMCID: PMC3546942
- DOI: 10.1186/1471-2407-12-609
Salvage chemoradiotherapy after primary chemotherapy for locally advanced pancreatic cancer: a single-institution retrospective analysis
Abstract
Background: There is no consensus on the indication for salvage chemoradiotherapy (CRT) after failure of primary chemotherapy for locally advanced pancreatic cancer (LAPC). Here we report on the retrospective analysis of patients who received salvage CRT after primary chemotherapy for LAPC. The primary objective of this study was to evaluate the efficacy and safety of salvage CRT after primary chemotherapy for LAPC.
Methods: Thirty patients who underwent salvage CRT, after the failure of primary chemotherapy for LAPC, were retrospectively enrolled from 2004 to 2011 at the authors' institution. All the patients had histologically confirmed pancreatic adenocarcinoma.
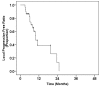
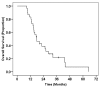
Results: Primary chemotherapy was continued until progression or emergence of unacceptable toxicity. Eventually, 26 patients (87%) discontinued primary chemotherapy because of local tumor progression, whereas four patients (13%) discontinued chemotherapy because of interstitial pneumonitis caused by gemcitabine. After a median period of 7.9 months from starting chemotherapy, 30 patients underwent salvage CRT combined with either S-1 or 5-FU. Toxicities were generally mild and self-limiting. Median survival time (MST) from the start of salvage CRT was 8.8 months. The 6 month, 1-year and 2-year survival rates from the start of CRT were 77%, 33% and 26%, respectively. Multivariate analysis revealed that a lower pre-CRT serum CA 19-9 level (≤ 1000 U/ml; p = 0.009) and a single regimen of primary chemotherapy (p = 0.004) were independent prognostic factors for survival after salvage CRT. The MST for the entire patient population from the start of primary chemotherapy was 17.8 months, with 2- and 3-year overall survival rates of 39% and 22%, respectively.
Conclusions: CRT had moderate anti-tumor activity and an acceptable toxicity profile in patients with LAPC, even after failure of gemcitabine-based primary chemotherapy. If there are any signs of failure of primary chemotherapy without distant metastasis, salvage CRT could be a treatment of choice as a second-line therapy. Patients with relatively low serum CA19-9 levels after primary chemotherapy may achieve higher survival rates after salvage CRT. The strategy of using chemotherapy alone as a primary treatment for LAPC, followed-by CRT with salvage intent should be further investigated in prospective clinical trials.
Trial registration: 2011-136
Figures

Similar articles
-
Gemcitabine and S-1 Induction Chemotherapy Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancers.Anticancer Res. 2017 Jan;37(1):233-237. doi: 10.21873/anticanres.11312. Anticancer Res. 2017. PMID: 28011497 Clinical Trial.
-
Phase II study of induction gemcitabine and S-1 followed by chemoradiotherapy and systemic chemotherapy using S-1 for locally advanced pancreatic cancer.Cancer Chemother Pharmacol. 2017 Jul;80(1):195-202. doi: 10.1007/s00280-017-3350-5. Epub 2017 Jun 8. Cancer Chemother Pharmacol. 2017. PMID: 28597040 Clinical Trial.
-
Phase 1 pharmacogenetic and pharmacodynamic study of sorafenib with concurrent radiation therapy and gemcitabine in locally advanced unresectable pancreatic cancer.Int J Radiat Oncol Biol Phys. 2014 Jun 1;89(2):284-91. doi: 10.1016/j.ijrobp.2014.02.024. Epub 2014 Apr 11. Int J Radiat Oncol Biol Phys. 2014. PMID: 24726286 Clinical Trial.
-
Gemcitabine and S-1 combination chemotherapy versus gemcitabine alone for locally advanced and metastatic pancreatic cancer: a meta-analysis of randomized controlled trials in Asia.J Chemother. 2015 Aug;27(4):227-34. doi: 10.1179/1973947815Y.0000000013. Epub 2015 Mar 20. J Chemother. 2015. PMID: 25790948
-
S-1 in the treatment of pancreatic cancer.World J Gastroenterol. 2014 Nov 7;20(41):15110-8. doi: 10.3748/wjg.v20.i41.15110. World J Gastroenterol. 2014. PMID: 25386059 Free PMC article. Review.
Cited by
-
Effects of chemoradiotherapy and chemotherapy on survival of patients with locally advanced pancreatic cancer: A meta-analysis of randomized controlled trials.Medicine (Baltimore). 2018 Sep;97(36):e12260. doi: 10.1097/MD.0000000000012260. Medicine (Baltimore). 2018. PMID: 30200163 Free PMC article. Review.
-
Prognostic factors in patients with locally advanced or borderline resectable pancreatic ductal adenocarcinoma: chemotherapy vs. chemoradiotherapy.Abdom Radiol (NY). 2021 Feb;46(2):655-666. doi: 10.1007/s00261-020-02661-w. Epub 2020 Aug 3. Abdom Radiol (NY). 2021. PMID: 32748250
-
Phase II Trial of Cetuximab and Conformal Radiotherapy Only in Locally Advanced Pancreatic Cancer with Concurrent Tissue Sampling Feasibility Study.Transl Oncol. 2014 Feb 1;7(1):55-64. doi: 10.1593/tlo.13724. eCollection 2014 Feb. Transl Oncol. 2014. PMID: 24772208 Free PMC article.
-
Concurrent Chemoradiotherapy Versus Chemotherapy Alone for Unresectable Locally Advanced Pancreatic Cancer: A Retrospective Cohort Study.Cancer Res Treat. 2016 Jul;48(3):1045-55. doi: 10.4143/crt.2015.226. Epub 2015 Oct 16. Cancer Res Treat. 2016. PMID: 26511805 Free PMC article.
-
ACTN4 copy number increase as a predictive biomarker for chemoradiotherapy of locally advanced pancreatic cancer.Br J Cancer. 2015 Feb 17;112(4):704-13. doi: 10.1038/bjc.2014.623. Epub 2015 Jan 20. Br J Cancer. 2015. PMID: 25602965 Free PMC article.
References
-
- Shinchi H, Takao S, Noma H, Matsuo Y, Mataki Y, Mori S, Aikou T. Length and quality of survival after external-beam radiotherapy with concurrent continuous 5-fluorouracil infusion for locally unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2002;53:146–150. doi: 10.1016/S0360-3016(01)02806-1. - DOI - PubMed
-
- Moertel CG, Frytak S, Hahn RG, O'Connell MJ, Reitemeier RJ, Rubin J, Schutt AJ, Weiland LH, Childs DS, Holbrook MA, Lavin PT, Livstone E, Spiro H, Knowlton A, Kalser M, Barkin J, Lessner H, Mann-Kaplan R, Ramming K, Douglas HO Jr, Thomas P, Nave H, Bateman J, Lokich J, Brooks J, Chaffey J, Corson JM, Zamcheck N, Novak JW. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::AID-CNCR2820480803>3.0.CO;2-4. - DOI - PubMed
-
- Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst. 1988;80:751–755. - PubMed
-
- Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, Fukushima M. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548–557. doi: 10.1097/00001813-199607000-00010. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

