Photoremovable protecting groups in chemistry and biology: reaction mechanisms and efficacy
- PMID: 23256727
- PMCID: PMC3557858
- DOI: 10.1021/cr300177k
Photoremovable protecting groups in chemistry and biology: reaction mechanisms and efficacy
Figures
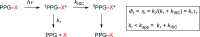


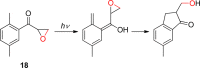
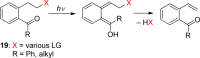
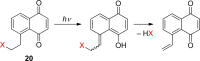
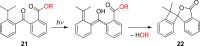
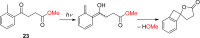
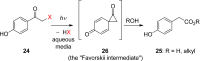
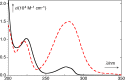
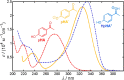
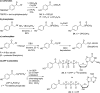
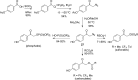
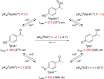
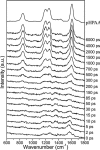
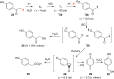


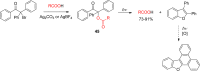
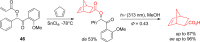

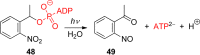


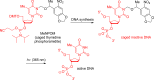
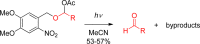
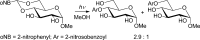
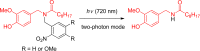


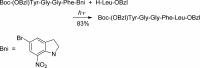
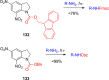

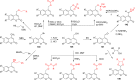
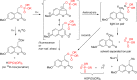

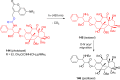




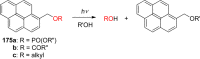
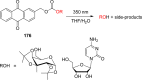

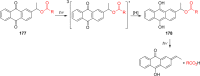
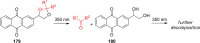
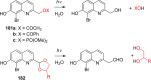
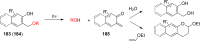
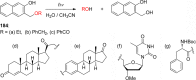
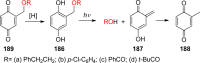
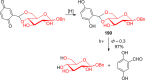
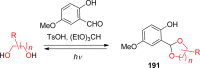
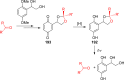
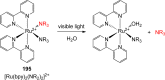



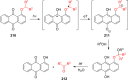
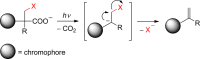
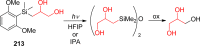
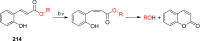










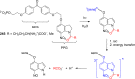
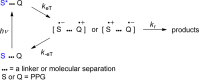
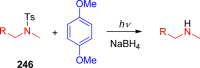
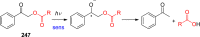
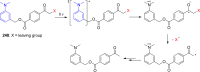
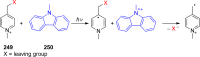

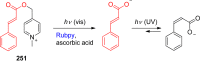
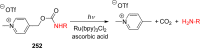







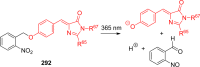

References
-
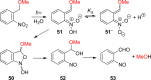
- Barltrop J. A.; Schofield P. Tetrahedron Lett. 1962, 16, 697.
-
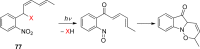
- Barton D. H. R.; Chow Y. L.; Cox A.; Kirby G. W. Tetrahedron Lett. 1962, 23, 1055.
- Barton D. H. R.; Chow Y. L.; Cox A.; Kirby G. W. J. Chem. Soc. 1965, 3571.
-
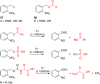
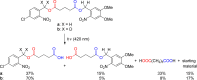
- Patchornik A.; Amit B.; Woodward R. B. J. Am. Chem. Soc. 1970, 92, 6333.
-
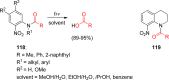
- Sheehan J. C.; Wilson R. M. J. Am. Chem. Soc. 1964, 86, 5277.
-
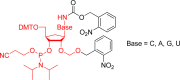
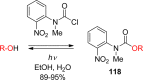
- Engels J.; Schlaeger E. J. J. Med. Chem. 1977, 20, 907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

