New insights into desmosome regulation and pemphigus blistering as a desmosome-remodeling disease
- PMID: 23257250
- PMCID: PMC11916772
- DOI: 10.1016/j.kjms.2012.08.001
New insights into desmosome regulation and pemphigus blistering as a desmosome-remodeling disease
Abstract
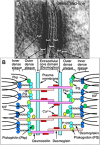
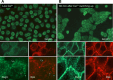
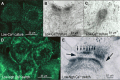
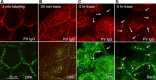
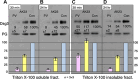
Desmosomes in keratinocytes are the most important intercellular adhering junctions that provide structural strength for the epidermis. These junctions are connected directly with desmosomal cadherin proteins. Desmosomal cadherins are divided into four desmogleins (Dsgs), Dsg1-4, and three desmocollins (Dscs), Dsc1-3, all of which are involved in desmosomal adhesion by homo- and/or heterophilic binding between Dsgs and Dscs in a Ca(2+)-dependent manner. Cadherins are present on the cell surface and anchor keratin intermediate filaments (KIFs) to their inner cytoplasmic surface to generate an intracellular KIF-skeletal scaffold through several associate proteins, including plakoglobin, plakophillin, and desmoplakins. As such, the desmosomal contacts between adjacent cells generate an intercellular KIF scaffold throughout the whole epidermal sheet. However, despite these critical roles in maintaining epidermal adhesion and integrity, desmosomes are not static structures. Rather, they are dynamic units that undergo regular remodeling, i.e., assembly and disassembly, to allow for cell migration within the epidermis in response to outside-in signaling during epidermal differentiation. Recently, two cell-cell adhesion states controlled by desmosomes have been recognized, including "stable hyperadhesion (Ca(2+)-independent)" and "dynamic weak-adhesion (Ca(2+)-dependent)" conditions. These conditions are mutually reversible through cell signaling events involving protein kinase C (PKC) and epidermal growth factor receptor. Pemphigus vulgaris (PV) is an autoimmune bullous disease caused by anti-Dsg3 antibodies. Binding of these antibodies to Dsg3 causes endocytosis of Dsg3 from the cell surface and results in the specific depletion of Dsg3 from desmosomes, an event linked to acantholysis in the epidermis. This binding of anti-Dsg3 antibody to Dsg3 in epidermal keratinocytes activates PKC, to generate the "weak-adhesion (Ca(2+)-dependent)" state of desmosomes. The weak-adhesion desmosomes appear to be the susceptible desmosomal state and a prerequisite for Dsg3 depletion from desmosomes, pivotal and specific events leading to PV blistering. These observations allow us to propose a concept for pemphigus blistering disorders as a "desmosome-remodeling impairment disease" involving a mechanism of Dsg3 nonassembly and depletion from desmosomes through PV immunoglobulin G-activated intracellular signaling events.
Copyright © 2012. Published by Elsevier B.V.
Figures







References
-
- Kitajima Y.. Mechanisms of desmosome assembly and disassembly. Clin Exp Dermatol. 2002; 27: 684–690. - PubMed
-
- He W., Cowin P., Stokes D.L.. Untangling desmosomal knots with electron tomography. Science. 2003; 302: 109–113. - PubMed
-
- Kottke M.D., Margaret D., Kottke E.D., Andrew P.K.. The desmosome: cell science lessons from human diseases. J Cell Sci. 2006; 119: 797–806. - PubMed
-
- North A.J., Bardsley W.G., Hyam J., Bornslaeger E.A., Cordingley H.C., Trinnaman B., et al. Molecular map of the desmosomal plaque. J Cell Sci. 1999; 112: 4325–4336. - PubMed
-
- Jonca N., Guerrin M., Hadjiolova K., Caubet C., Gallinaro H., Simon M., et al. Corneodesmosin, a component of epidermal corneocyte desmosomes, displays homophilic adhesive properties. J Biol Chem. 2001; 277: 5024–5029. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

